What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Solved We begin by showing that the compressibility factor

As the pressure approaching zero i.e., very low pressure, the curves plotted between compressibility factor Z and P n mole of gases have the following characteristics.I. The intercept on the y-axis leads
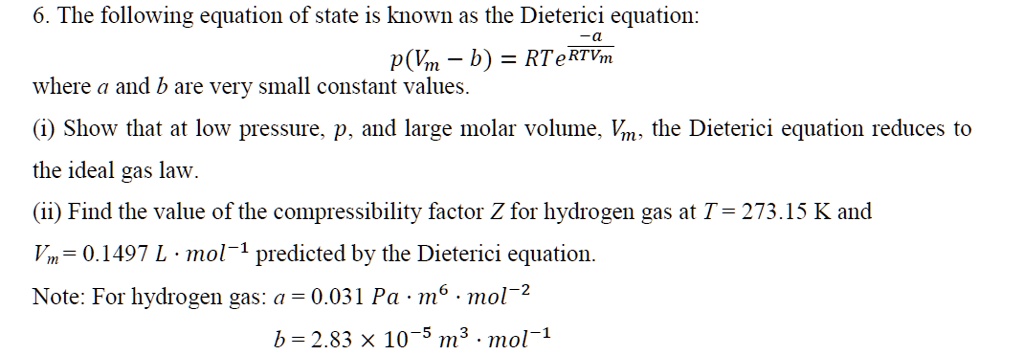
SOLVED: The following equation of state is known as the Dieterici equation: p(Vm - b) = RT * e^(RT/Vm), where a and b are very small constant values. Show that at low

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
Calculate the critical constants of a gas whose van der Waals constant

Compressibility Factor Calculator

At a high pressure, the compressibility factor (Z) of a real gas is us

Compressibility factor (gases) - Citizendium

At high pressure, the compressibility factor for one mole of van der w

SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect

Answered: 4. Determine expressions for the…

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
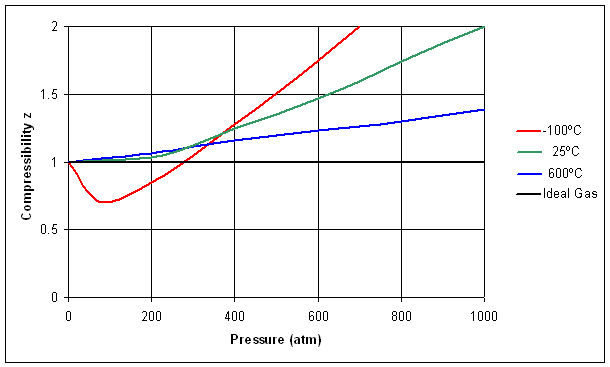
Gas Laws – First Year General Chemistry






