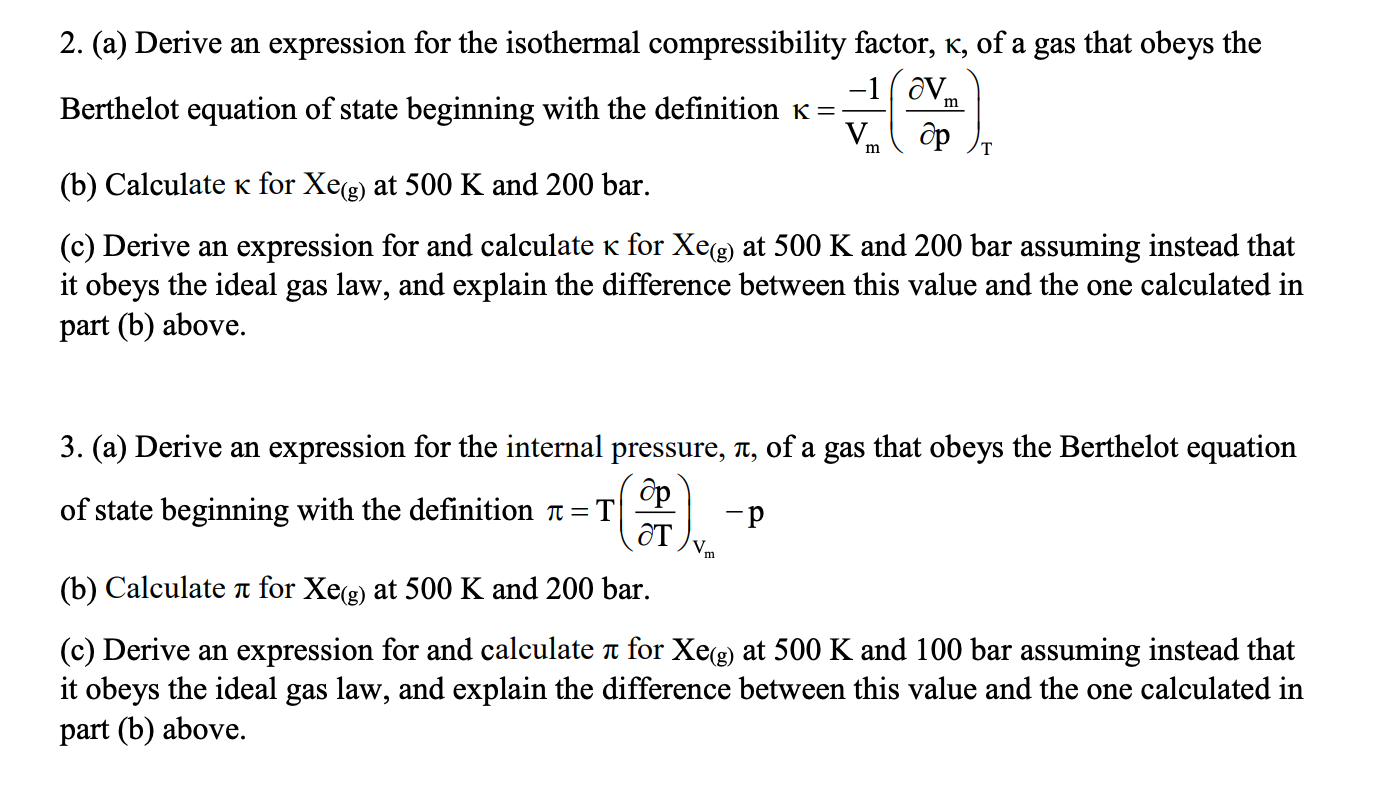
Solved] Why is the compressibility factor less than 1 at most
4.8
(279)
Write Review
More
$ 19.99
In stock
Description
Answer to Why is the compressibility factor less than 1 at most conditions?

Solved The compressibility factor, Z, can be thought of as a

Deviation Of Real Gas From Ideal Gas Behavior

physical chemistry - Is the compressibility factor smaller or

3.2 Real gas and compressibility factor – Introduction to

The compressibility factor (Z) of real gas is usually less than 1 at l

Compressibility factor - Wikipedia

The value of compressibility factor at the critical state the gas

If Z is a compressibility factor, van der Waals equation at low
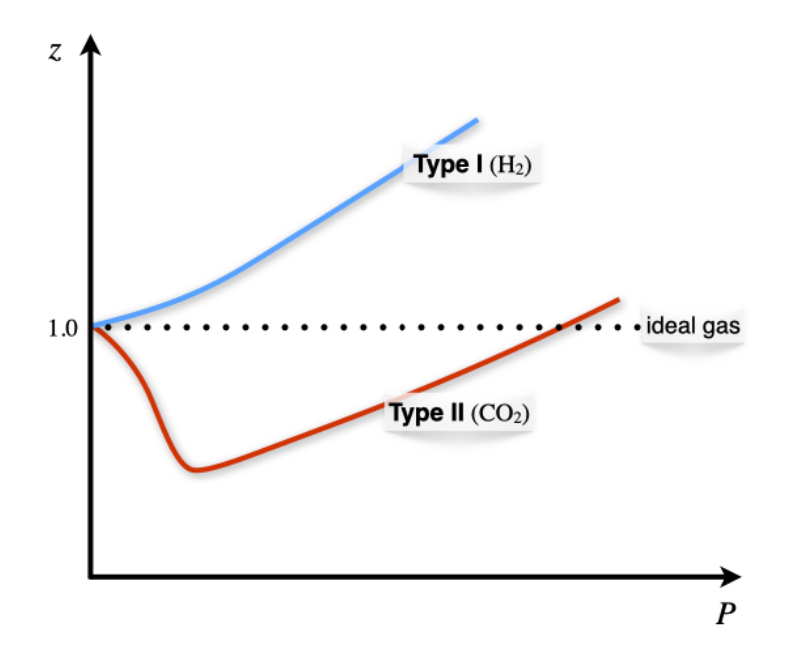
11.3: Critical Phenomena - Chemistry LibreTexts

Determine Compressibility of Gases
Related products
You may also like