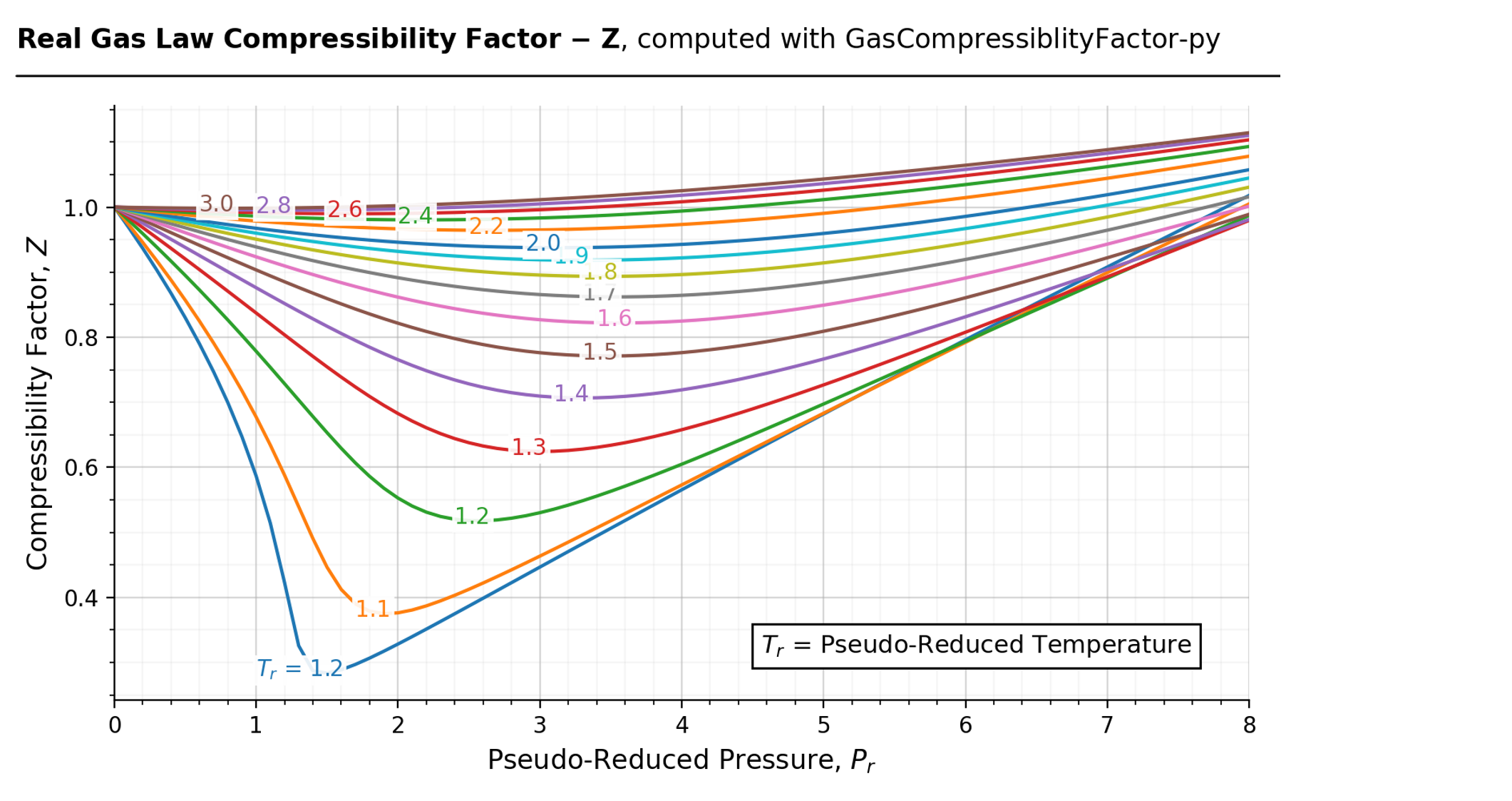
Compressibility factor (Z) for a van der Waals real gas at
Share your videos with friends, family and the world

PDF) A Modified Form of the van der Waals Equation of State
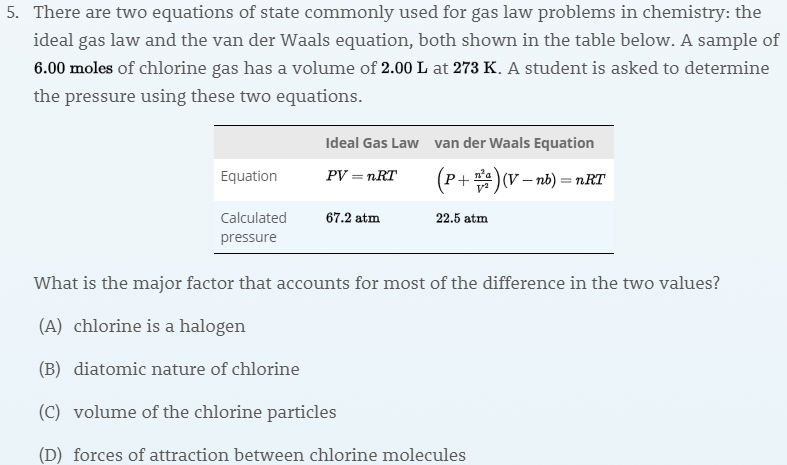
What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?
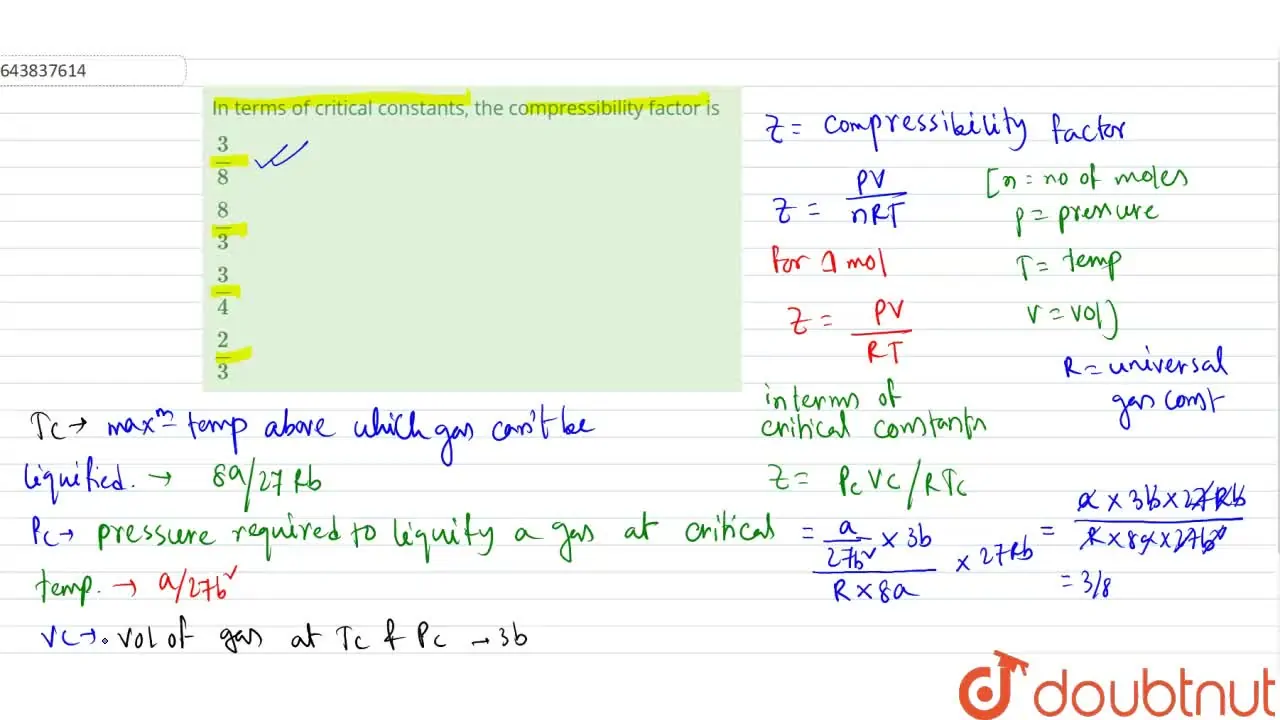
Bengali] In terms of critical constants, the compressibility factor i

The compressibility factor a real gas high pressure is:-1 - frac{Pb} {RT}1 + frac {RT} {Pb}11 + frac {Pb} {RT}
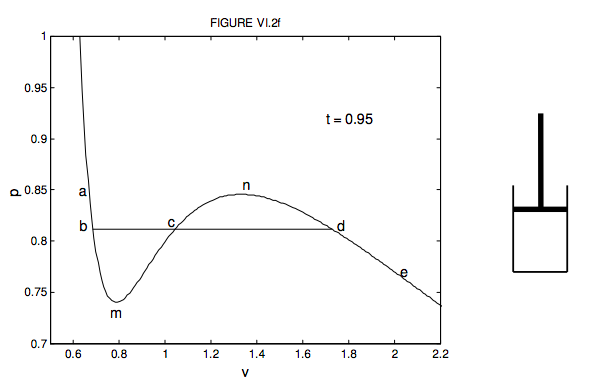
6.3: Van der Waals and Other Gases - Physics LibreTexts

Isenthalpic point and real gas compressibility relation
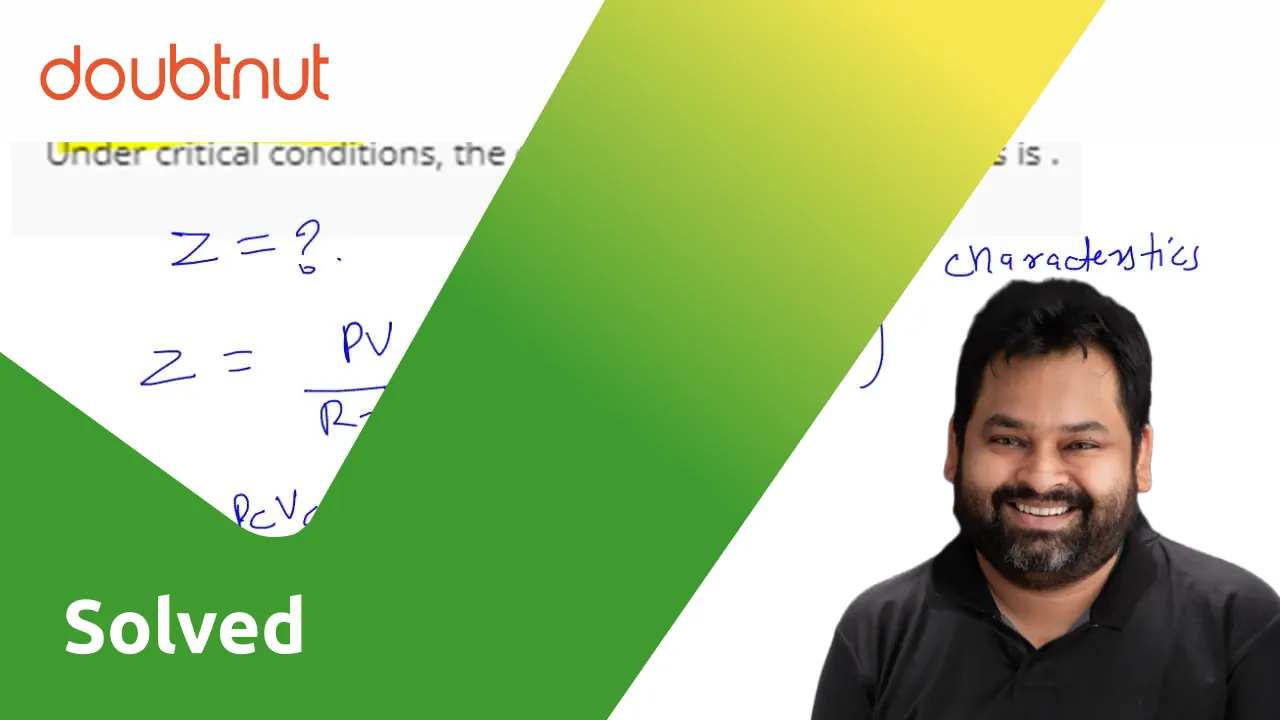
Under critical conditions, the compressibility factor for a gas is .
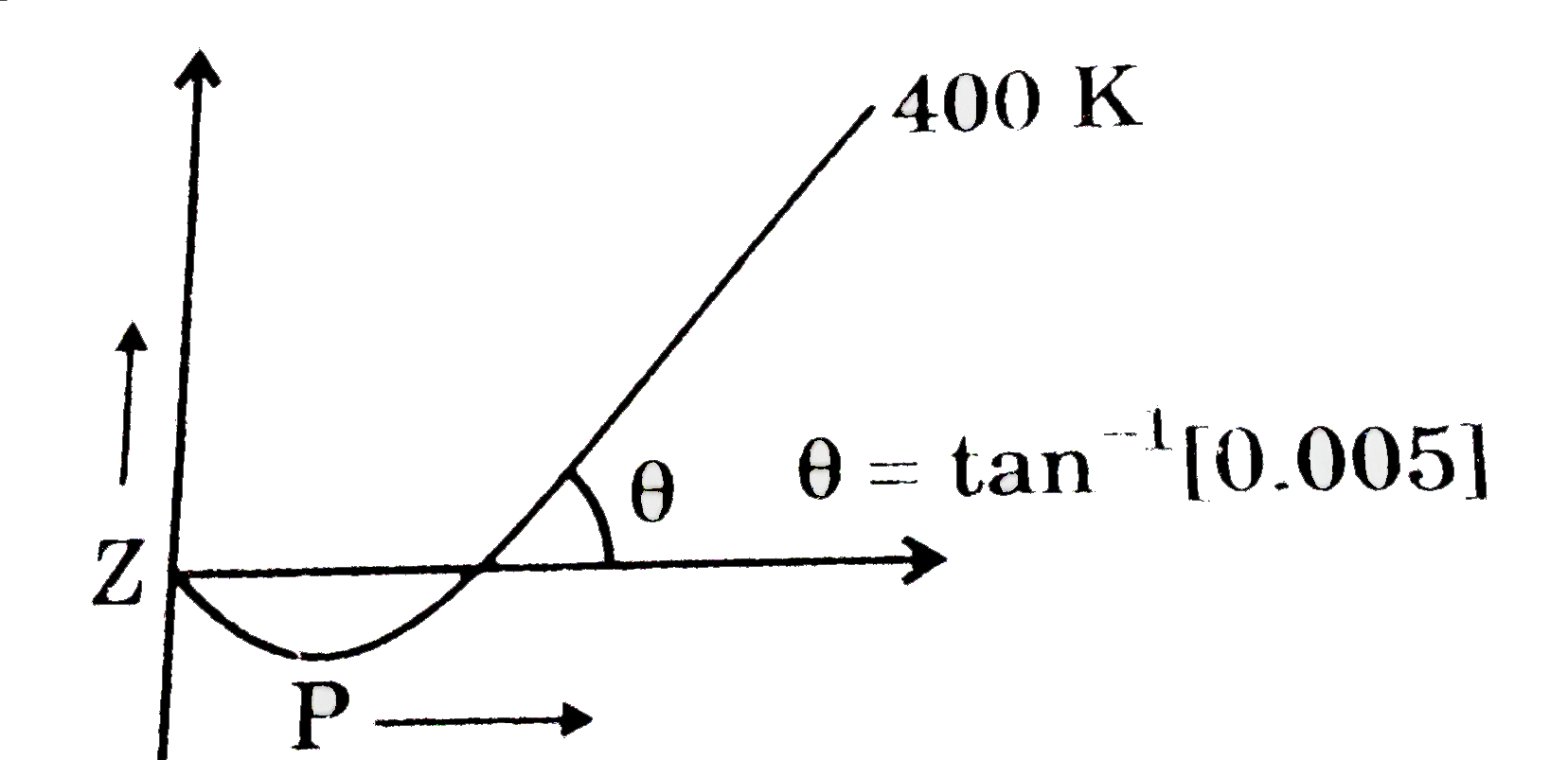
What will be the nature of forces at critical conditions for a real ga

SOLVED: The fugacity of a van der Waals gas can be determined using the expression for the compressibility factor Z. The expression for fugacity of a van der Waals gas is given

Gas Compressibility Factor and Control Valve Sizing

For a real gas (mol.mass =60) if density at critical point is 0.80g//c
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions