physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
4.7
(523)
Write Review
More
$ 20.50
In stock
Description
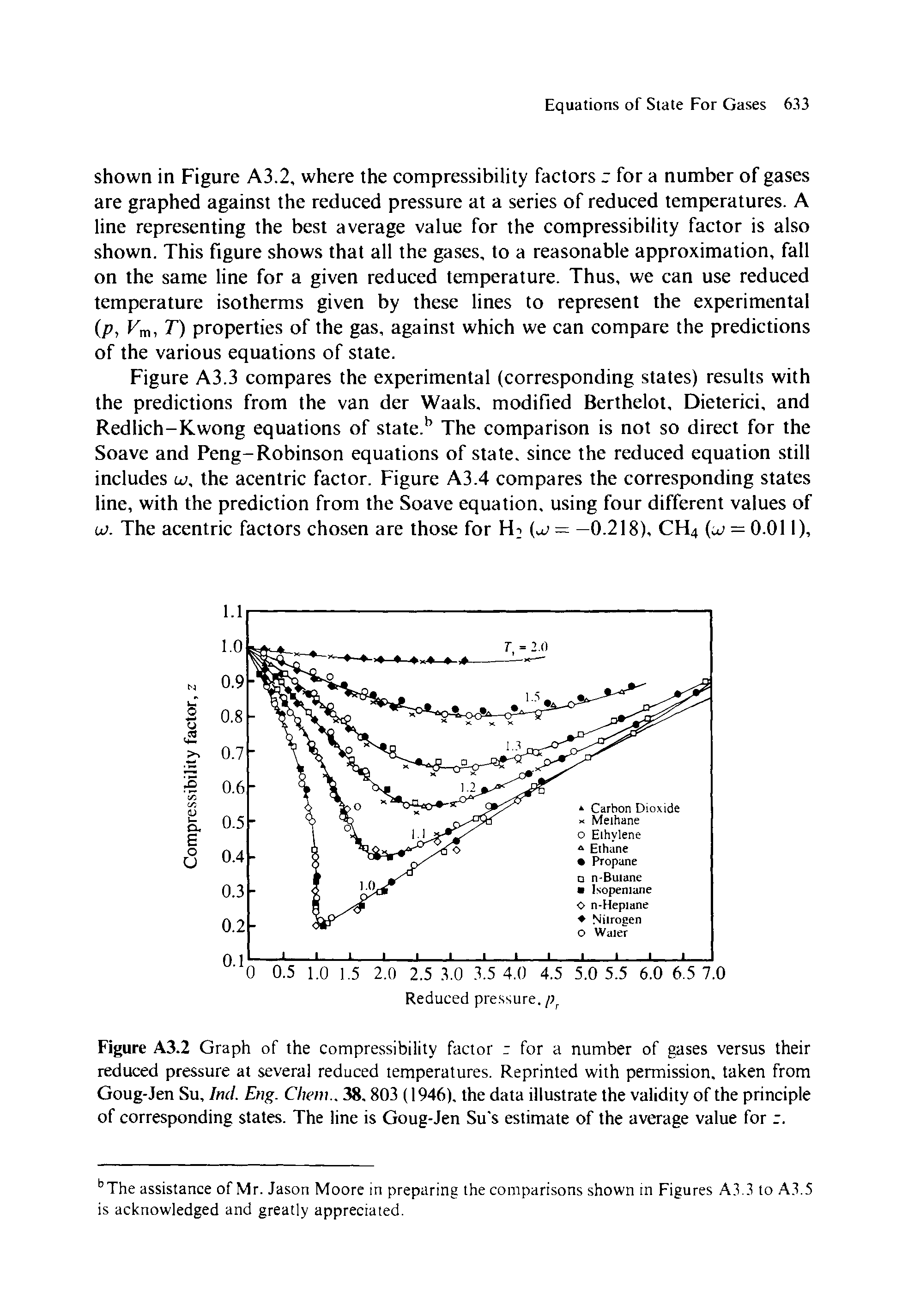
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m
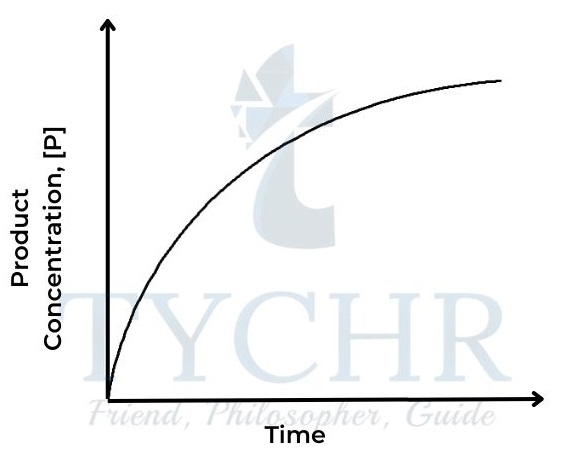
IB Chemistry, Chemical Kinetics Notes

The Conversion of Carbon Monoxide and Carbon Dioxide by

Net-zero emissions chemical industry in a world of limited

physical chemistry - Compressibility Factor Graph - Which gas

Partial Pressure- Formula, Dalton's Law, Mixture of Ideal Gas

Chemical vapor deposition of 2D materials: A review of modeling

Non-Ideal Gas Behavior Chemistry: Atoms First

Gas - Wikipedia
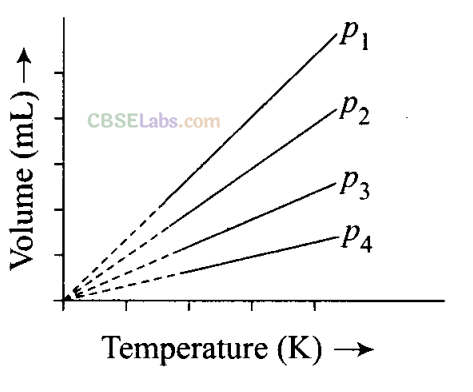
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter
Related products
You may also like