What is the compressibility factor (Z) for 0.02 mole of a van der Waal
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

What is the compressibility factor (Z) for 0.02 mole of a van der

Van Der Waals Equation - an overview
SOLVED: The compression factor Z reveals information about

ODPOOD B-76.& What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given : RT =
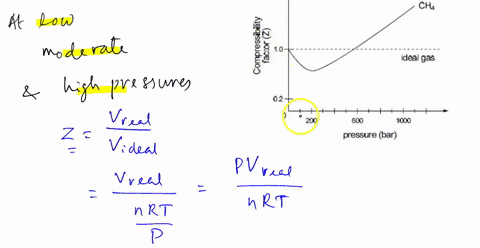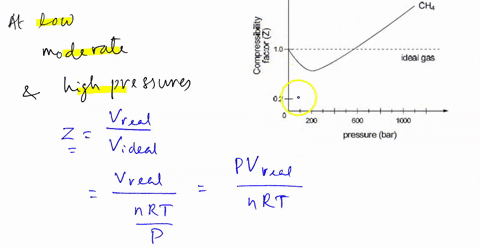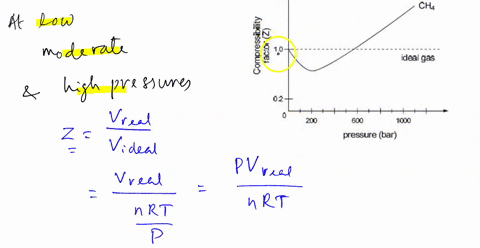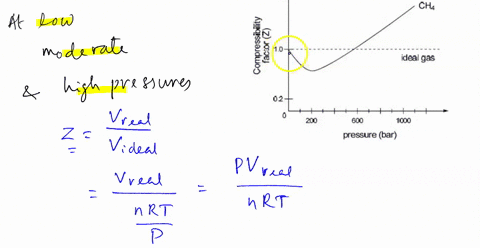
Punjabi] The compressibillity of a gas is greater than unity at 1 atm
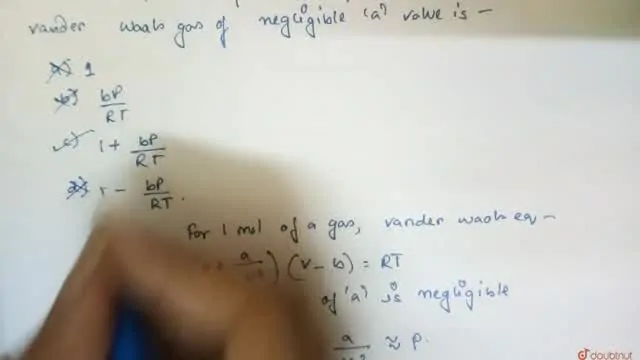
Bengali] The compresibility factor (Z) of one mole of a van der waals

SOLVED: If PR=3, TR=2.0, the compressibility factor (Z) is equal

al Gases f.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given: RT =

Punjabi] What is the compressibility factor (Z) for 0.02 mole of a va

02 mole of a van der Waals gas pressure of 0.1 alin. Civanges unpredictably (B-16. What is the compressibility factor (Z) 0.02 mole of a Assume the size of gas molecules is
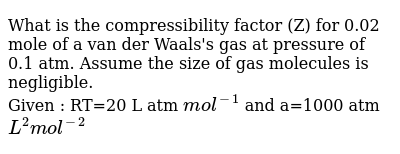
What is the compressibility factor (Z) for 0.02 mole of a van der Waal

Filo Student Questions For CBSE , Grade 9 , Chemistry