
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Compressibility factor Z is plotted against pressure p for four

Multi-scale simulation of wave propagation and liquefaction in a
1642646504_391987.png

The given graph represents the variation of Z (compressibility

Compressibility Factor Z Important Concepts and Tips for JEE Main
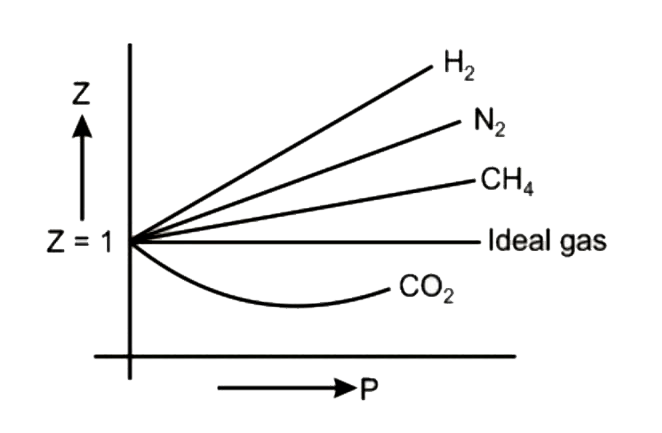
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

01 Gaseous State#### PDF, PDF, Gases

gaseous state

Determine Compressibility of Gases
What does a compressibility factor >1 signify, apart from a deviation from the ideal gas behaviour? Is it more compressible? - Quora
What is compressibility factor (Z)? - Sarthaks eConnect

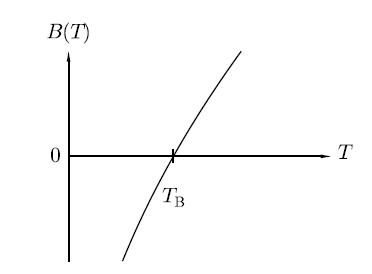
Boyle's temperature or Boyle point is the temperature which a real






/product/98/119185/1.jpg?4805)


