
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Where is the deviation of a gas from ideal behaviour maximum at? - Quora

Compressibility factor - Wikipedia

y factor Compressibility factor 2 V is plotted agalnst pressure RT What is the correct order of correct order of liquet ability of the gases shown in the above graphi (5) Hz

Compressibility factorZPVnRTis plotted againstpressure What is the correct order of liquefiability of the gases shown in the above graph

PVCompressibility factor Z=-isnRTplotted against pressure: What isthe correct order of liquefiabilityof the

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Non-Ideal Gas Behavior Chemistry: Atoms First
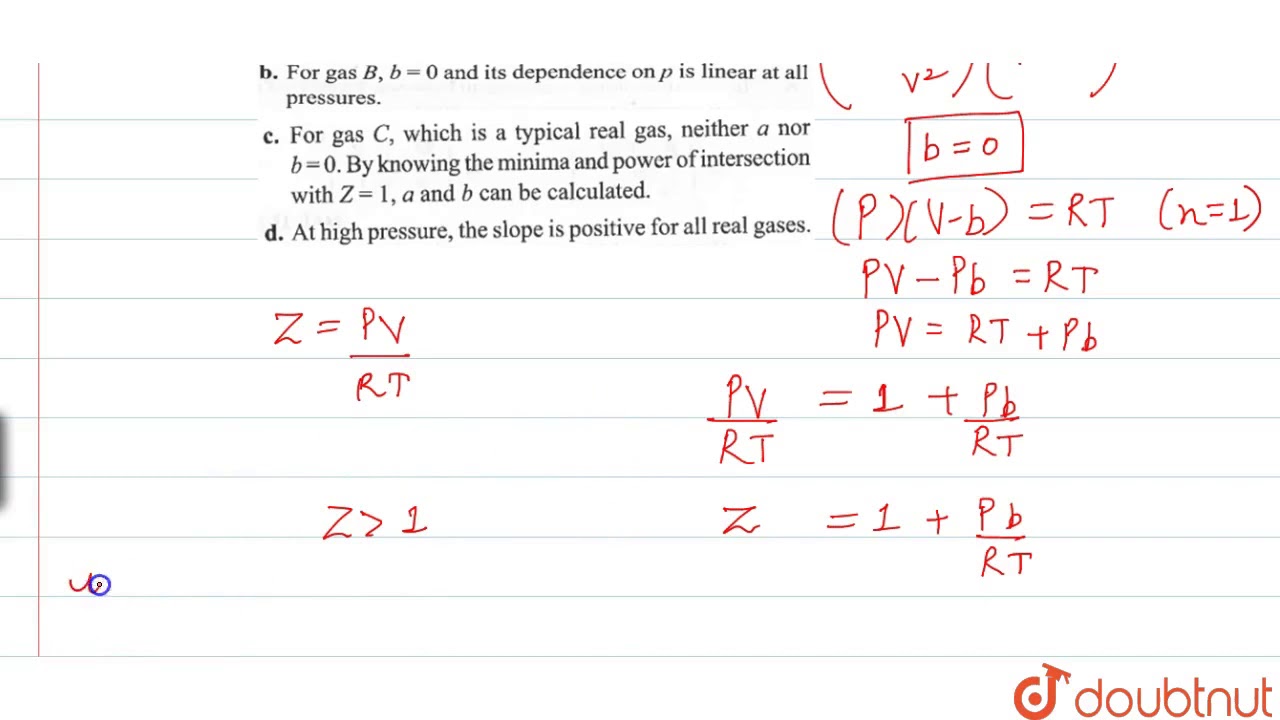
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

Gas—General - ScienceDirect








