
OneClass: For a real gas, the compressibility factor, Z, is

Compressibility factor - Wikipedia

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor Z - Gaseous State

For a gas at a given temperature, the compression factor is described by the empirical equa - OneClass

OneClass: #1 The Joule-Thomson inversion locus is the set of points for which the Joule-Thomson coeff
Ideal gases and real gases are compressible or not compressible what is the compressible factor for real gases and ideal gases.

Compressibility factor - Wikipedia

Real Gas Behavior The Compression Factor (Z) [Example #2]
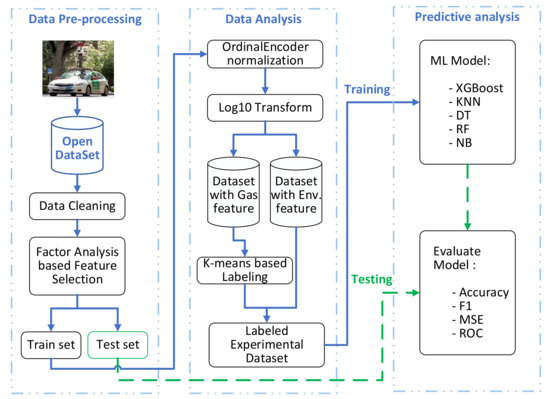
Applied Sciences, Free Full-Text

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal
If z<1, does it mean that the gases behave more like perfect or real gases? - Quora

Compressibility factor (gases) - Knowino

Deviation Of Real Gas From Ideal Gas Behavior

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect








