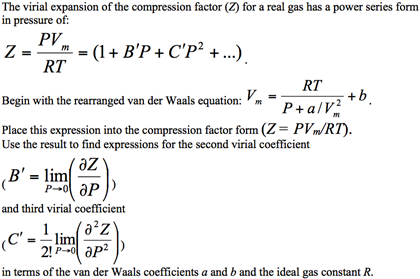
Compression Factor Exam Problem using Molar Volumes - Fully Explained!

Compressibility factor - Wikipedia

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

Solved APPENDIX Problem 1: Molar Volume and Compressibility

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

The Compression Factor, Z, and Real Gases - What you NEED to Know
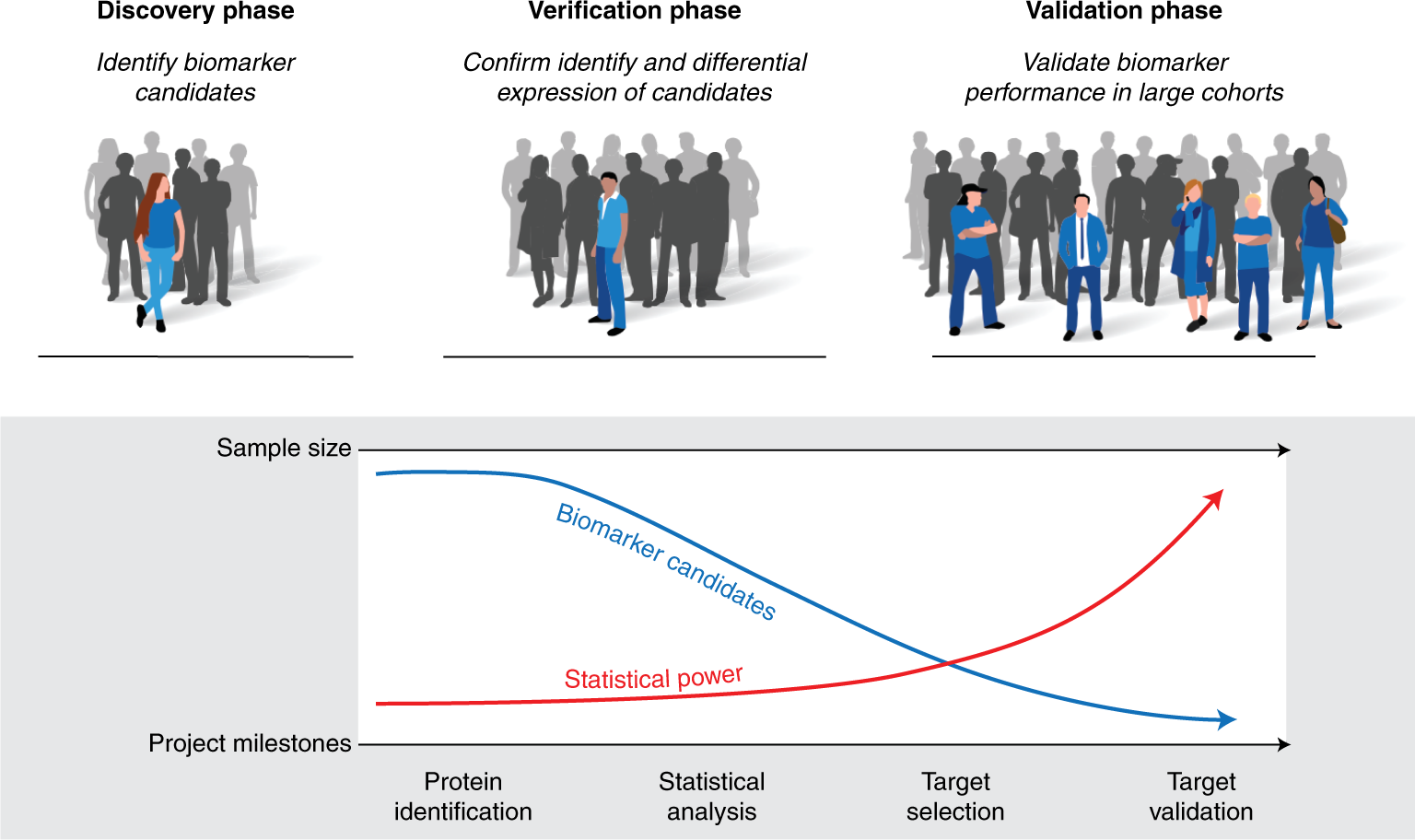
Tutorial: best practices and considerations for mass-spectrometry-based protein biomarker discovery and validation

Determining the Work Done by an Isothermal Process., Chemistry
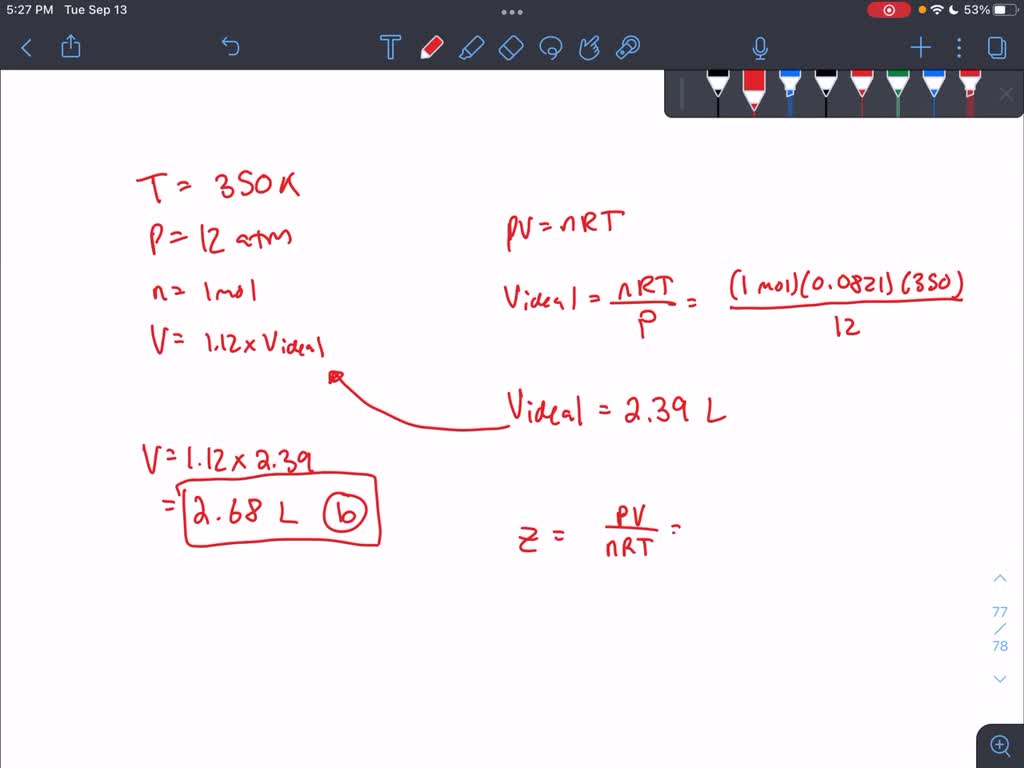
SOLVED: A gas at 350 K and 12 atm has a molar volume 12 per cent larger than that calculated from the perfect gas law. Calculate (a) The compression factor under these
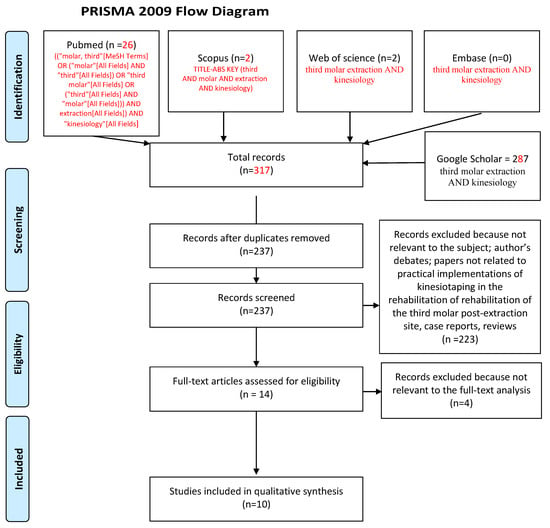
JCM, Free Full-Text
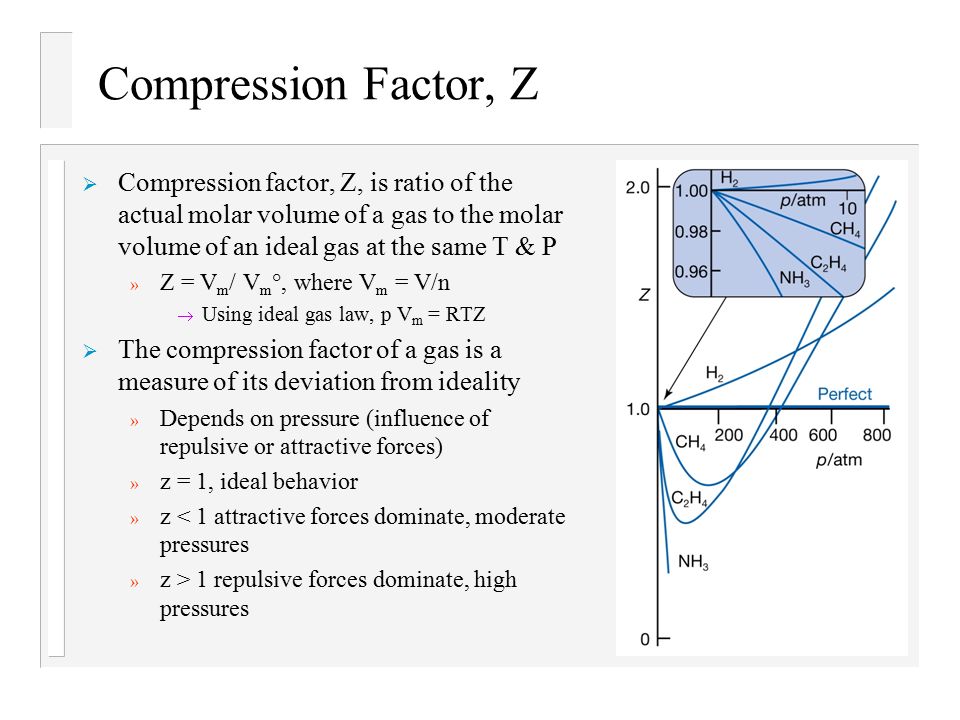
Text: Physical Chemistry, 7th Edition, Peter Atkins and J. de Paula - ppt download

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at