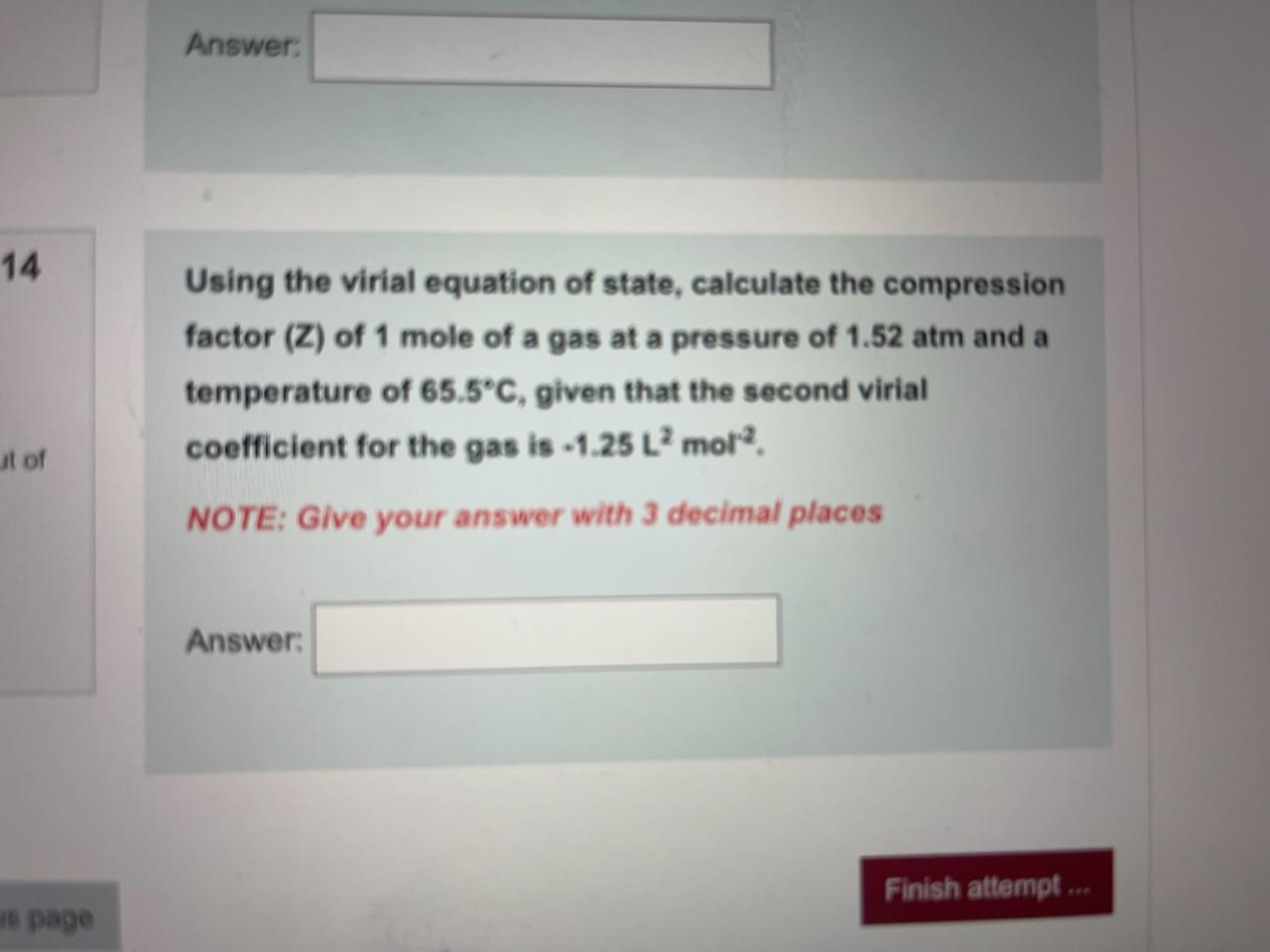
Solved Using the virial equation of state, calculate the

OneClass: Calculate Z and V for ethylene at 25degree C (298.15 K) and 12 bar by the following equatio
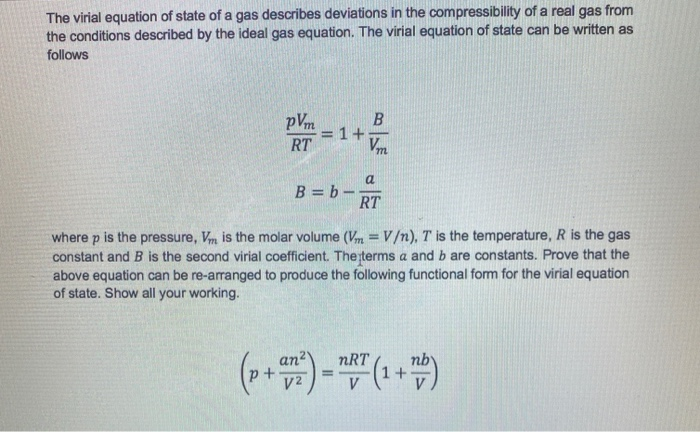
Solved The virial equation of state of a gas describes

Virial Equation of State Introduction
Finding the equations of state via the fundamental relation
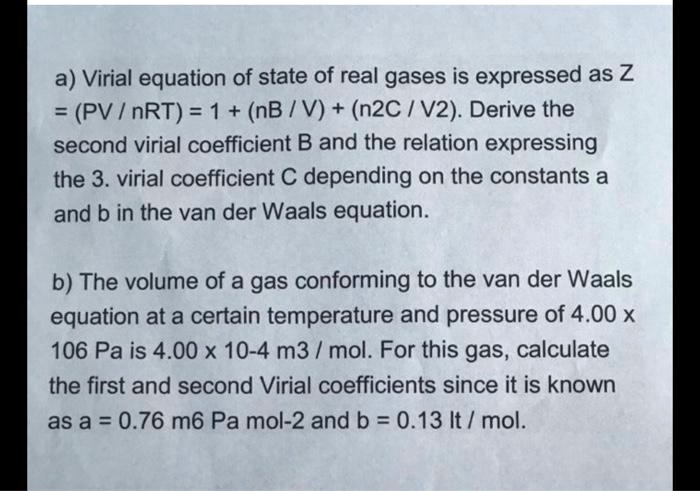
SOLVED: a) Virial equation of state of real gases is expressed as

Second Virial Coefficient of the Equation of State - an overview

The virial equation for nitrogen gas is PV=RT(1+4.41×10-4P). Using the virial equation of state for
If the third virial coefficient of He is 4×10^ 2 then calculate the volume of 2 mole He at STP
Equation of state - Wikipedia

PDF) Adequacy of the virial equation of state and cluster expansion
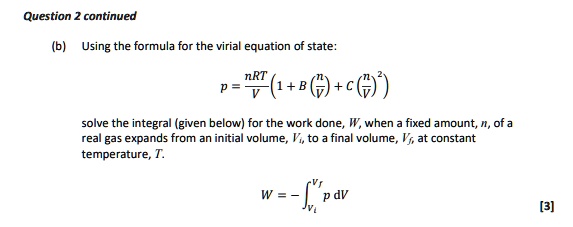
SOLVED: Question continued Using the formula for the virial equation of state: p = (1+b@+c() solve the integral (given below) for the work done; W, when fixed amount; , of a real
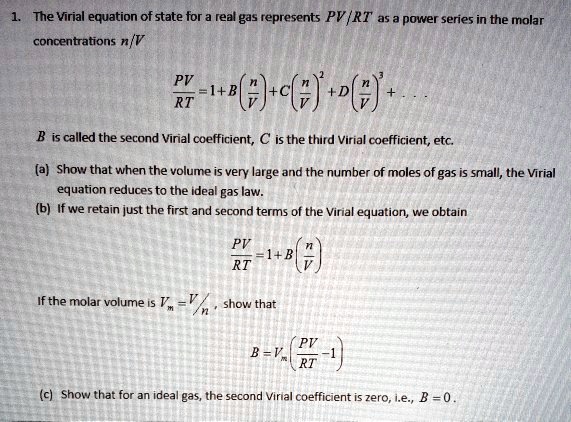
SOLVED: The Virial equation of state for a real gas represents PV = RT (1 + B/V + C/V^2 + ), where B is called the second Virial coefficient, C is the

SOLVED: The Virial equation of state is the most theoretically sound of the equations of state we have discussed so far: RT = 1 ± √(B(T) + C(T)). In this equation, B(T)