The entropy change for the conversion of 36 g water to vapour at
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
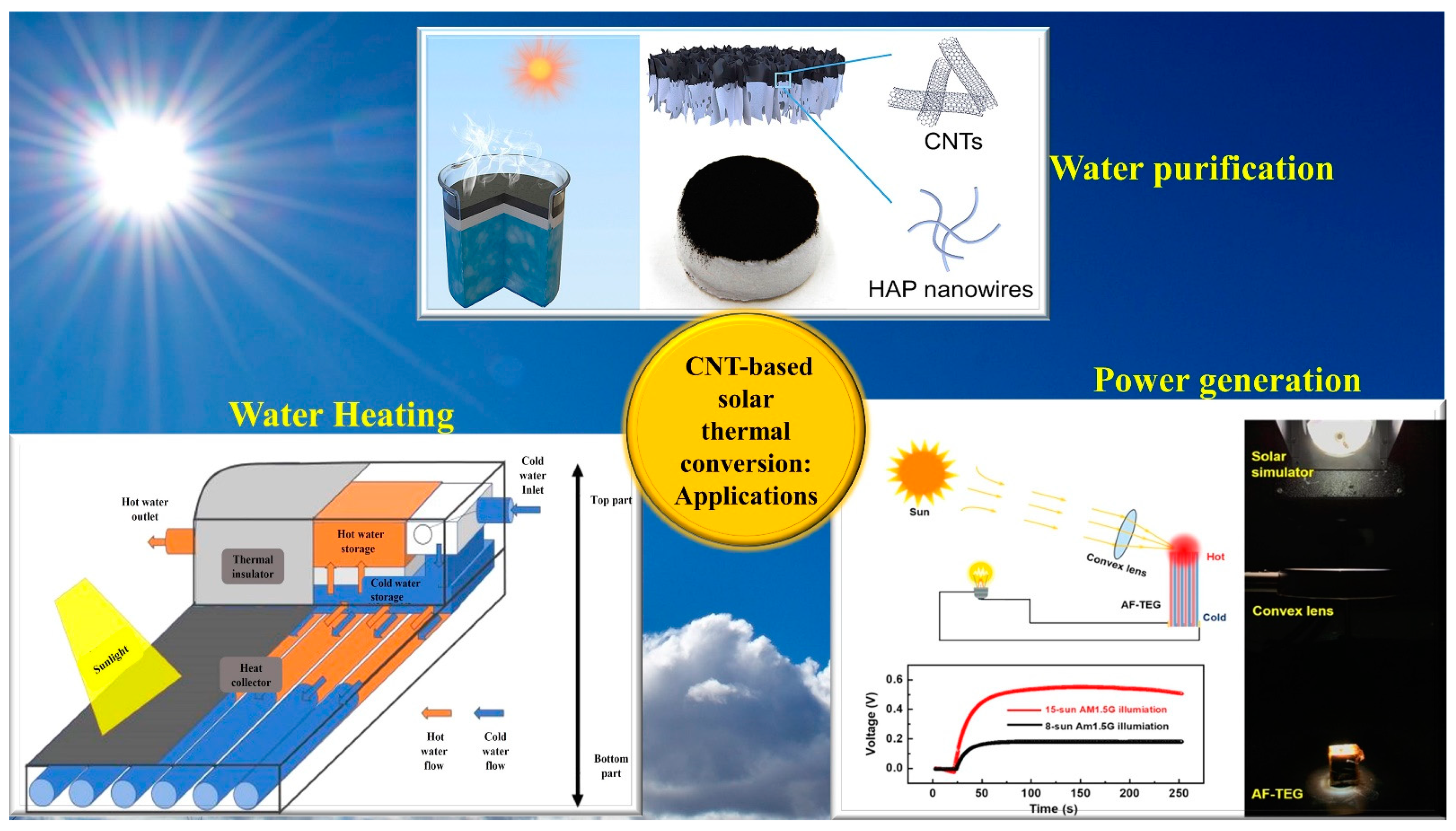
Nanomaterials, Free Full-Text

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is
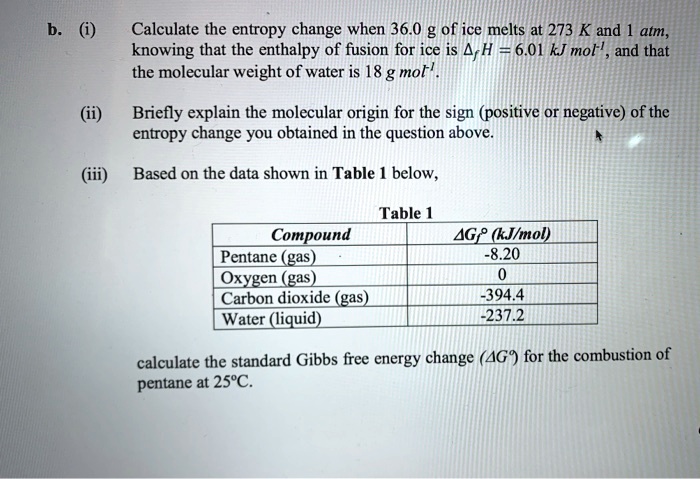
SOLVED: (i) Calculate the entropy change when 36.0 g of ice melts at 273 K and atm , knowing that the enthalpy of fusion for ice is 4H = 6.01 kJ molr

The constant volume heat capacity of liquid CO 2 ( d = 1.1663 g/cm 3

What is the entropy change (in JK^(-1)mol^(-1)) when one mole of ice i
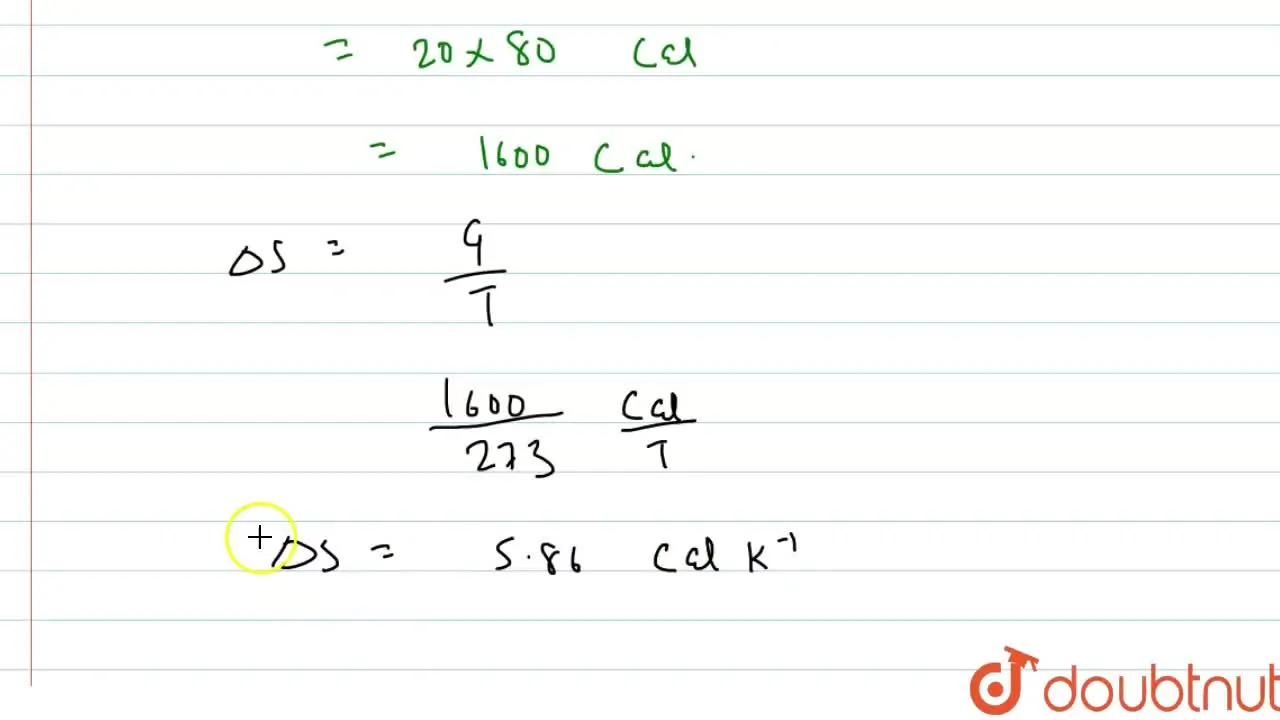
Calculate the entropy change when 20.0 g of ice changes to liquid wate
What is the entropy change in going from vapour to liquid state at any temperature? - Quora

Calculate the entropy change in surroundings when 1.00 mol of H2O (l) is formed under standard.

Phase change material-based thermal energy storage - ScienceDirect



)





