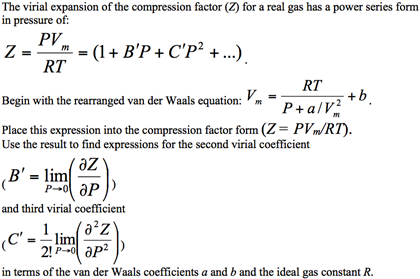
Pick only the incorrect statement.for gas A, a=0,the
Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases
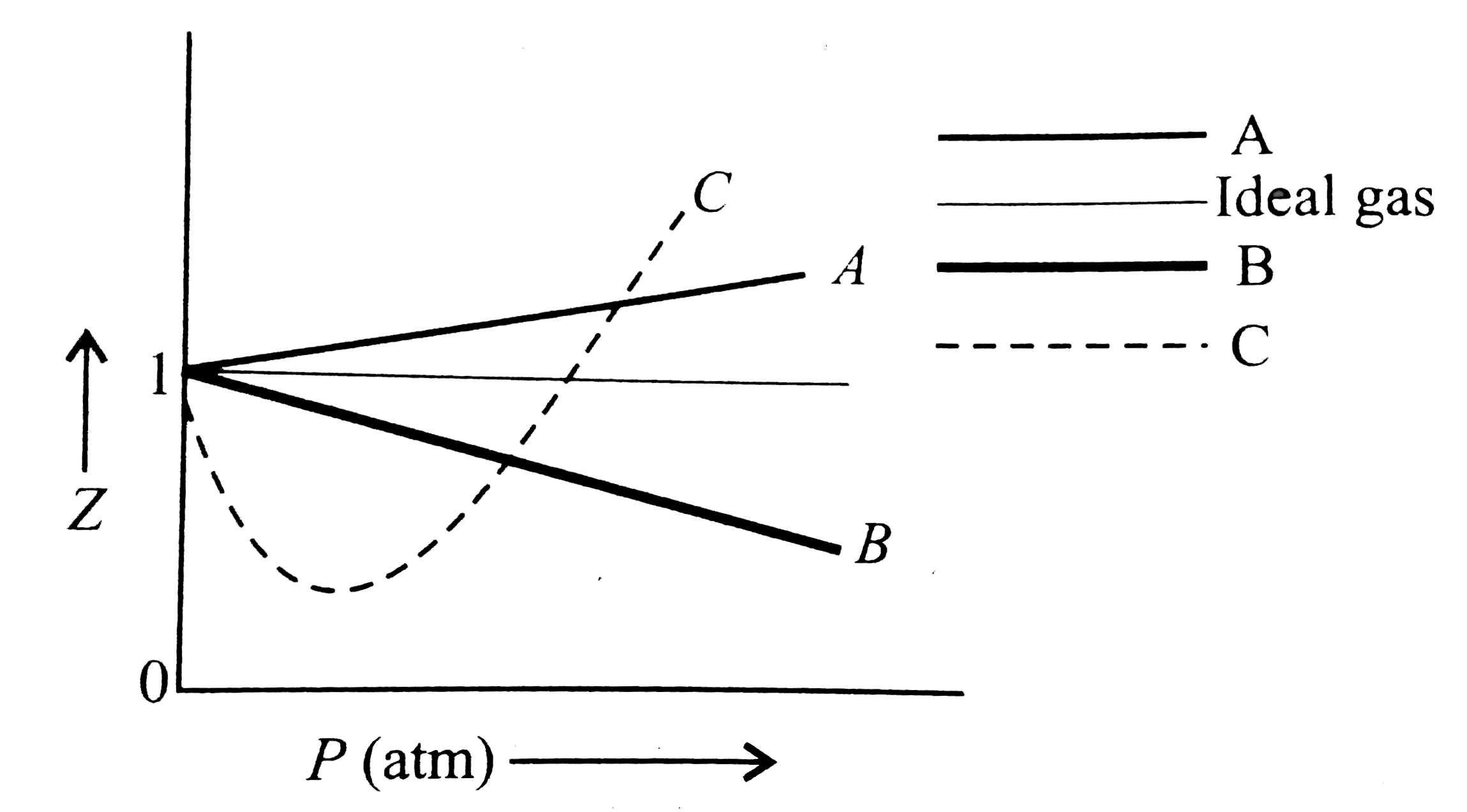
For gas C, which is a typical real gas, neither a nor b=0. By knowing
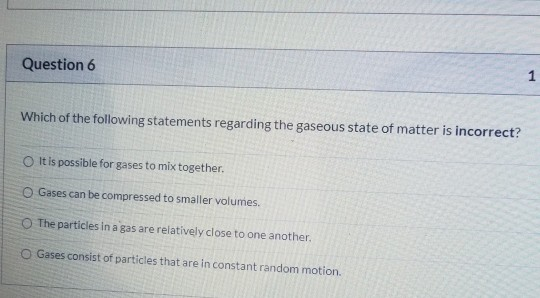
Solved Question 6 1 Which of the following statements
a= Van der Waal's constant for pressure correction b= Van der

Solved For the reaction: N2(g) + 3H2(g) + 2NH3(g) AH = -92
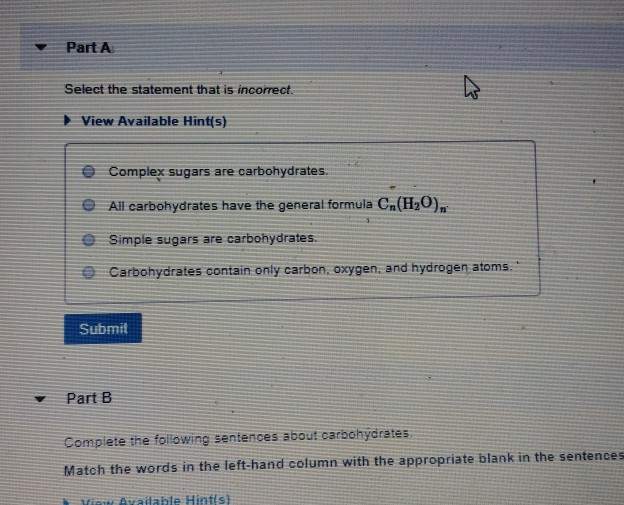
Solved Part A Select the statement that is incorrect. View
:max_bytes(150000):strip_icc()/prospecttheory.asp-FINAL-55296758049c4501808f54b52747cb35.png)
Prospect Theory: What It Is and How It Works, With Examples

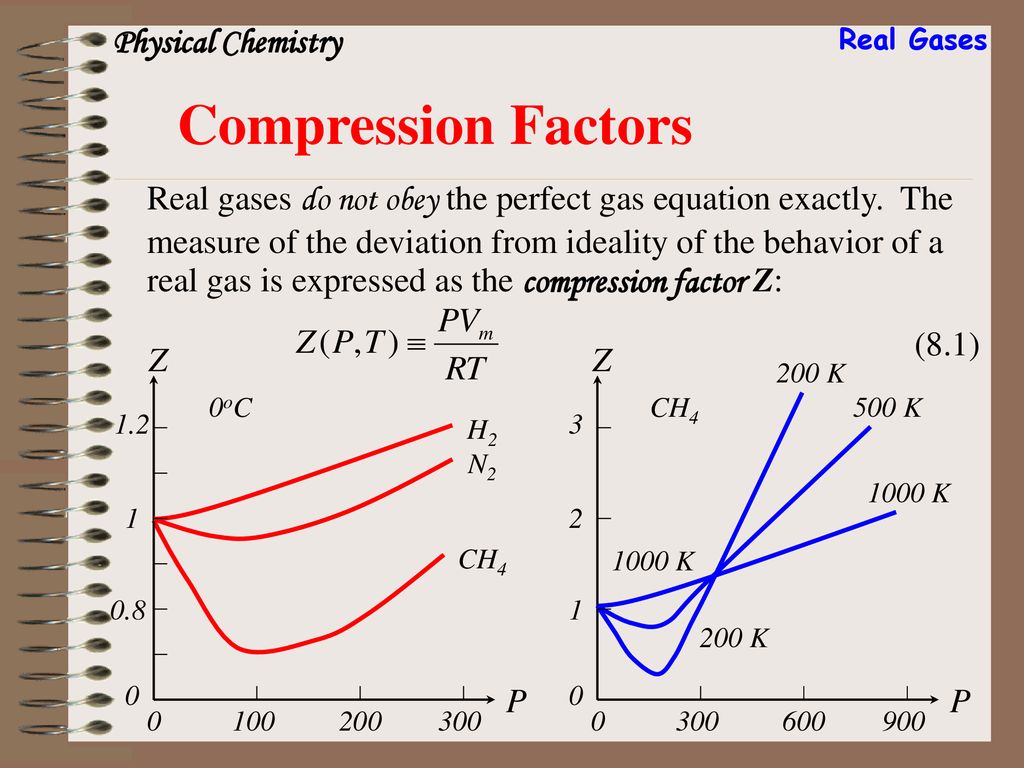
The given graph represents the variation of compressibility factor
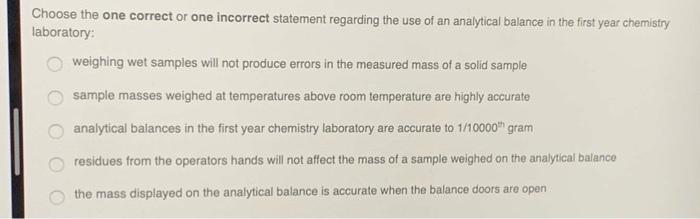
Solved Choose the one correct or one incorrect statement

Solved Which statement about free energy is INCORRECT? a. A