
The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions - Women's Healthcare
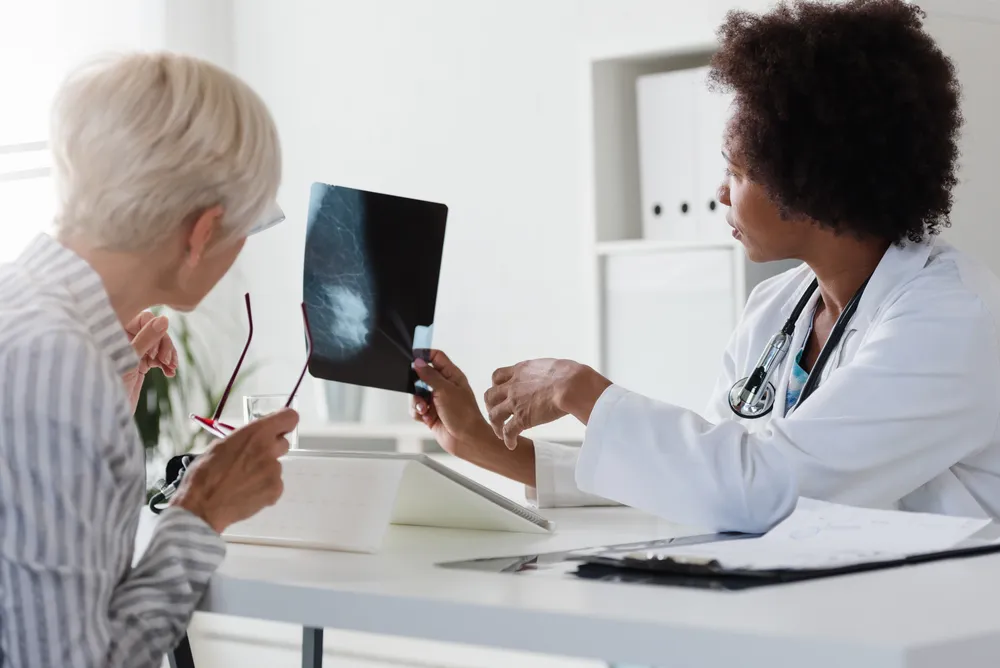
The FDA's Rule Change Requiring Providers to Inform Women About Breast Density Could Lead to a Flurry Of Questions – ActiveBeat – Your Daily Dose of Health Headlines

Breast cancer screening – News, Research and Analysis – The Conversation – page 1

FDA rule change requires mammogram centers to notify patients of breast density

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions - UPMC & Pitt Health Sciences News Blog

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News

FDA Will Require Dense Breast Disclosure at Mammogram Clinics - The New York Times

FDA dense breast rule promotes earlier cancer detection

N.S. news: Liberals push for more breast cancer testing

The FDA's Rule Change Requiring Providers to Inform Women About Breast Density Could Lead to a Flurry Of Questions – ActiveBeat – Your Daily Dose of Health Headlines

Early Signs of Osteopenia – ActiveBeat – Your Daily Dose of Health Headlines

DenseBreast-info, Inc.
The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions

FDA issues final rule on breast density notifications

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions
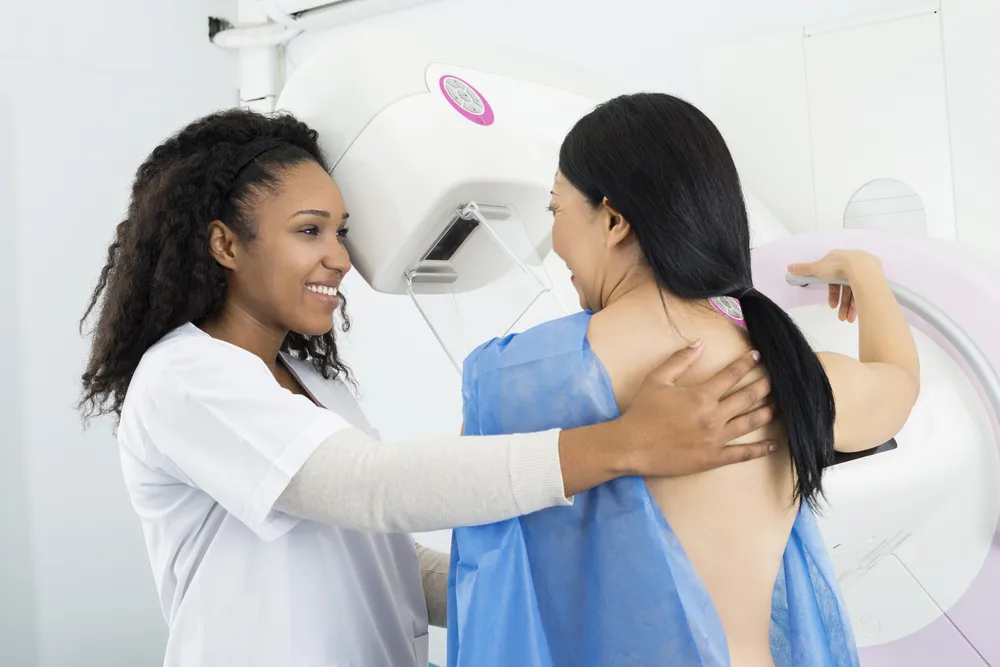
What to Know Before Your First Mammogram – ActiveBeat – Your Daily Dose of Health Headlines









