At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

Van der Waals Equation - Concept, Physics

The Van der Waals equation a real gas is : (P + 2) (V – b) = RT
Van der Waals Equation, Virial Expansion

p+a/v²) (v-b) = RT In the above 'van der waal equation', find the
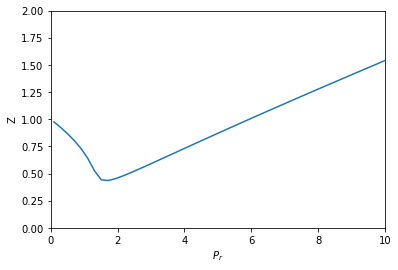
Compressibility factor variation from the van der Waals equation

3) Zone refining Cupellation Compressibility factor of carbon dioxide gas 0°C under low pressure is equal to 1148. (2) Pb (1) 1 (2) RT a (3) 1 RTV As 1-RT 49. Which of the following radicals is least stable?


The van der Waals equation 1 mole of gas isleft(p+dfrac{a}{V^{2
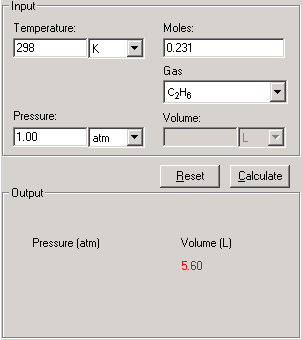
Real Gases - Van der Waals Equation

The Van Der Waals Equation, PDF, Gases
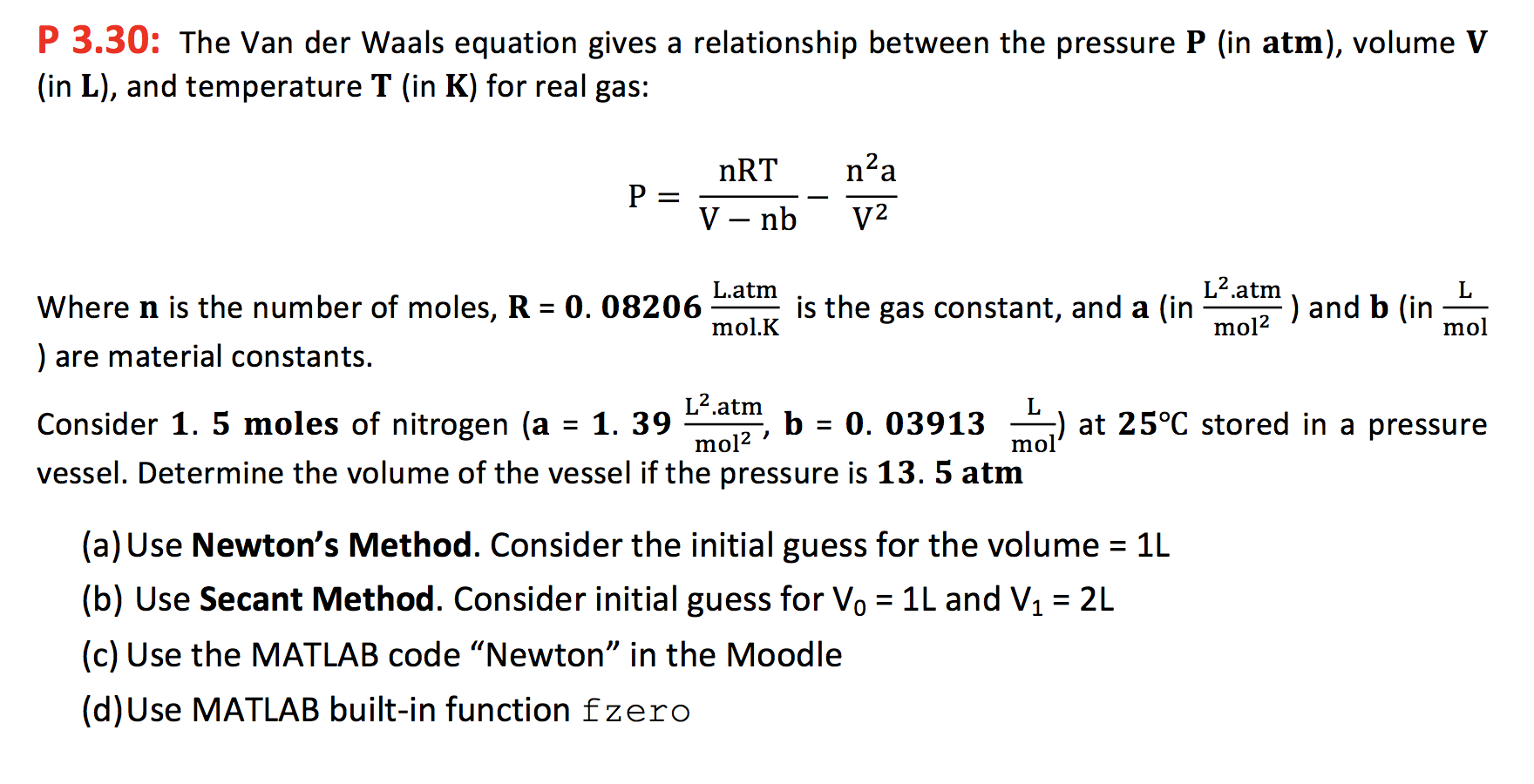
P 3.30: The Van der Waals equation gives a







