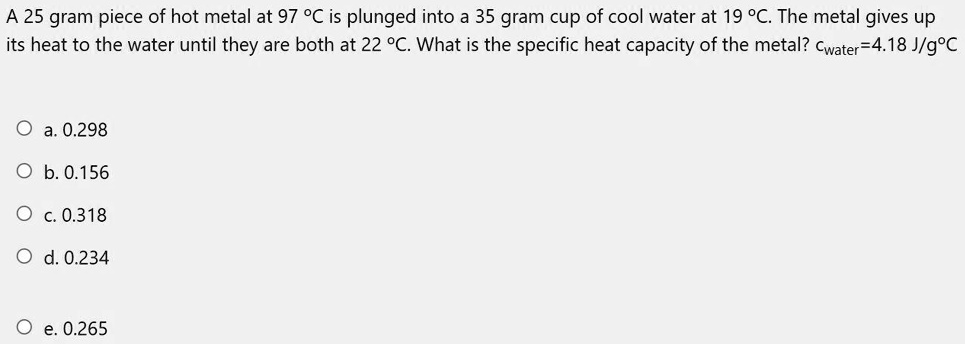
SOLVED: A 25 gram piece of hot metal at 97°C is plunged into a 35

A 25.0 g sample of metal at 16.0 °C is warmed to 22.1 °C by 259

AP Specific Heat (Final Temp. Metal Dropped into Water)

⏩SOLVED:Suppose that 25 g of each substance is initially at 27.0

Solved 4. Suppose a hot (89.5°C) piece of copper metal (c

Lockheed Martin F-35 Lightning II - Wikipedia

Solved 1. Calculate the amout of heat water absorbs from a
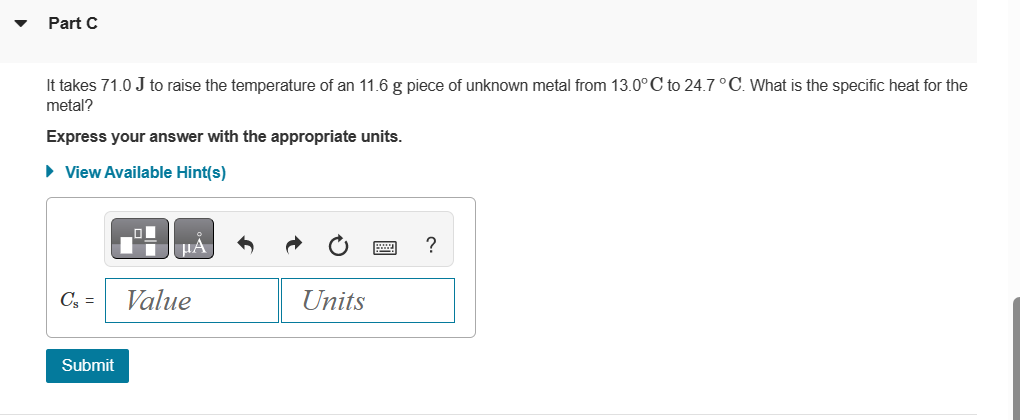
Solved The molar heat capacity of silver is 25.35 J/mol⋅∘C.

Solved 31. A piece of metal weighing 418.6 grams was put

The Works of Voltaire, Vol. XIX (Philosophical Letters)

SOLVED: a 4.50-g sample of copper metal at 25.0 C is heated by the

Steel Times International November December 2022 by Quartz Business Media - Issuu
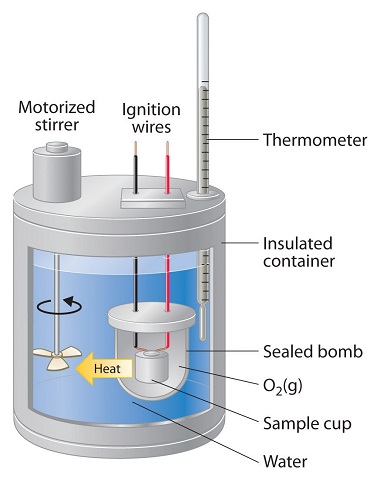
12.3: Heat Capacity, Enthalpy, and Calorimetry - Chemistry LibreTexts

The 100 Best Movies of All Time: Critics' Picks

Chemical method of water flow measurement in open channels









