Postembryonic RNAi in Heterorhabditis bacteriophora: a nematode insect parasite and host for insect pathogenic symbionts, BMC Developmental Biology
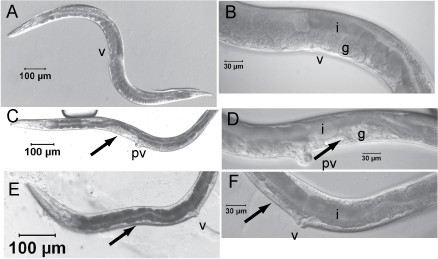
Background Heterorhabditis bacteriophora is applied throughout the world for the biological control of insects and is an animal model to study interspecies interactions, e.g. mutualism, parasitism and vector-borne disease. H. bacteriophora nematodes are mutually associated with the insect pathogen, Photorhabdus luminescens. The developmentally arrested infective juvenile (IJ) stage nematode (vector) specifically transmits Photorhabdus luminescens bacteria (pathogen) in its gut mucosa to the haemocoel of insects (host). The nematode vector and pathogen alone are not known to cause insect disease. RNA interference is an excellent reverse genetic tool to study gene function in C. elegans, and it would be useful in H. bacteriophora to exploit the H. bacteriophora genome project, currently in progress. Results Soaking L1 stage H. bacteriophora with seven dsRNAs of genes whose C. elegans orthologs had severe RNAi phenotypes resulted in highly penetrant and obvious developmental and reproductive abnormalities. The efficacy of postembryonic double strand RNA interference (RNAi) was evident by abnormal gonad morphology and sterility of adult H. bacteriophora and C. elegans presumable due to defects in germ cell proliferation and gonad development. The penetrance of RNAi phenotypes in H. bacteriophora was high for five genes (87–100%; Hba-cct-2, Hba-daf-21, Hba-icd-1; Hba-nol-5, and Hba-W01G7.3) and moderate for two genes (usually 30–50%; Hba-rack-1 and Hba-arf-1). RNAi of three additional C. elegans orthologs for which RNAi phenotypes were not previously detected in C. elegans, also did not result in any apparent phenotypes in H. bacteriophora. Specific and severe reduction in transcript levels in RNAi treated L1s was determined by quantitative real-time RT-PCR. These results suggest that postembryonic RNAi by soaking is potent and specific. Conclusion Although RNAi is conserved in animals and plants, RNAi using long dsRNA is not. These results demonstrate that RNAi can be used effectively in H. bacteriophora and can be applied for analyses of nematode genes involved in symbiosis and parasitism. It is likely that RNAi will be an important tool for functional genomics utilizing the high quality draft H. bacteriophora genome sequence.

Transcriptomic analysis of the entomopathogenic nematode Heterorhabditis bacteriophora TTO1, BMC Genomics

Insect Immunity to Entomopathogenic Nematodes and Their Mutualistic Bacteria
Silencing of Aphid Genes by dsRNA Feeding from Plants

RNA Processing, PDF, Rna Splicing

Identification of candidate infection genes from the model entomopathogenic nematode Heterorhabditis bacteriophora, BMC Genomics
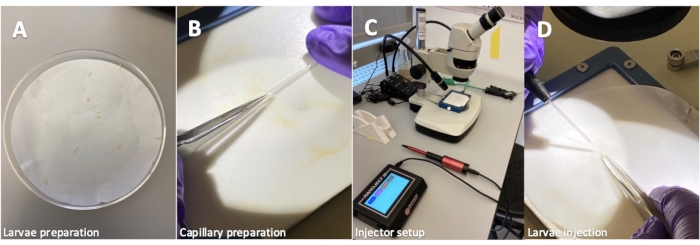
Drosophila melanogaster Larva Injection Protocol

Molecular Regulators of Entomopathogenic Nematode–Bacterial Symbiosis

RNA Processing, PDF, Rna Splicing

RNA interference in nematodes and the chance that favored Sydney Brenner, Journal of Biology








