The compressibility factor is Z = PV/R_g T. Evaluate
Answer to The compressibility factor is Z = PV/R_g T. Evaluate

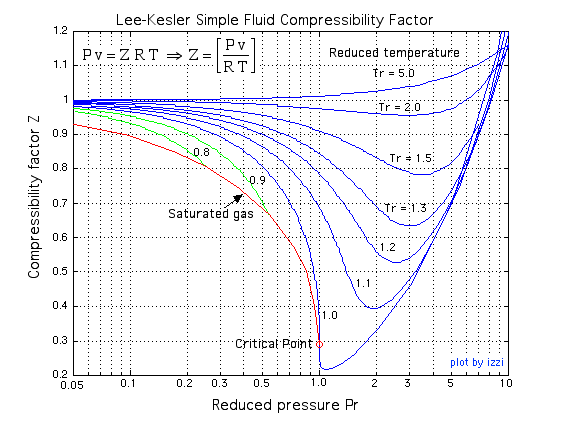
3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
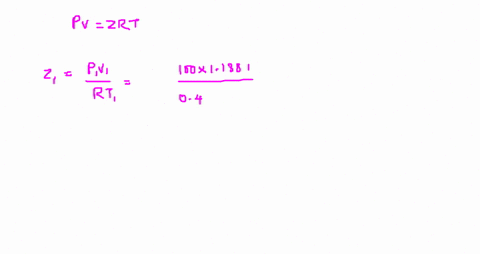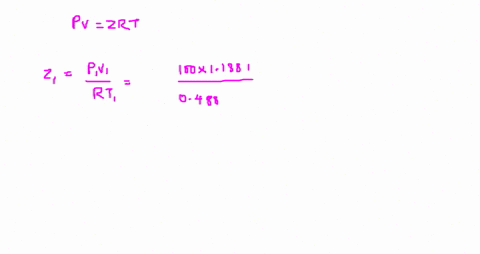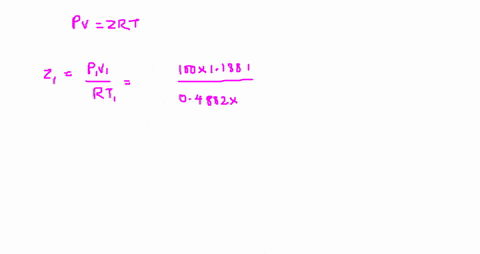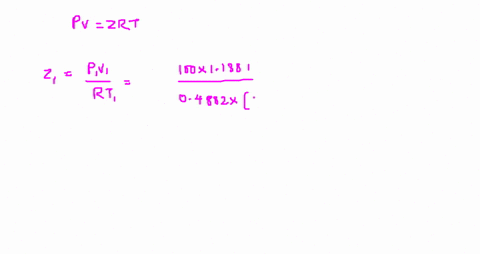
⏩SOLVED:The value of compressibility factor (Z) for this vapour is?…
What is compressibility factor? - Quora

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia

EGR 334 Thermodynamics Chapter 3: Section ppt video online download
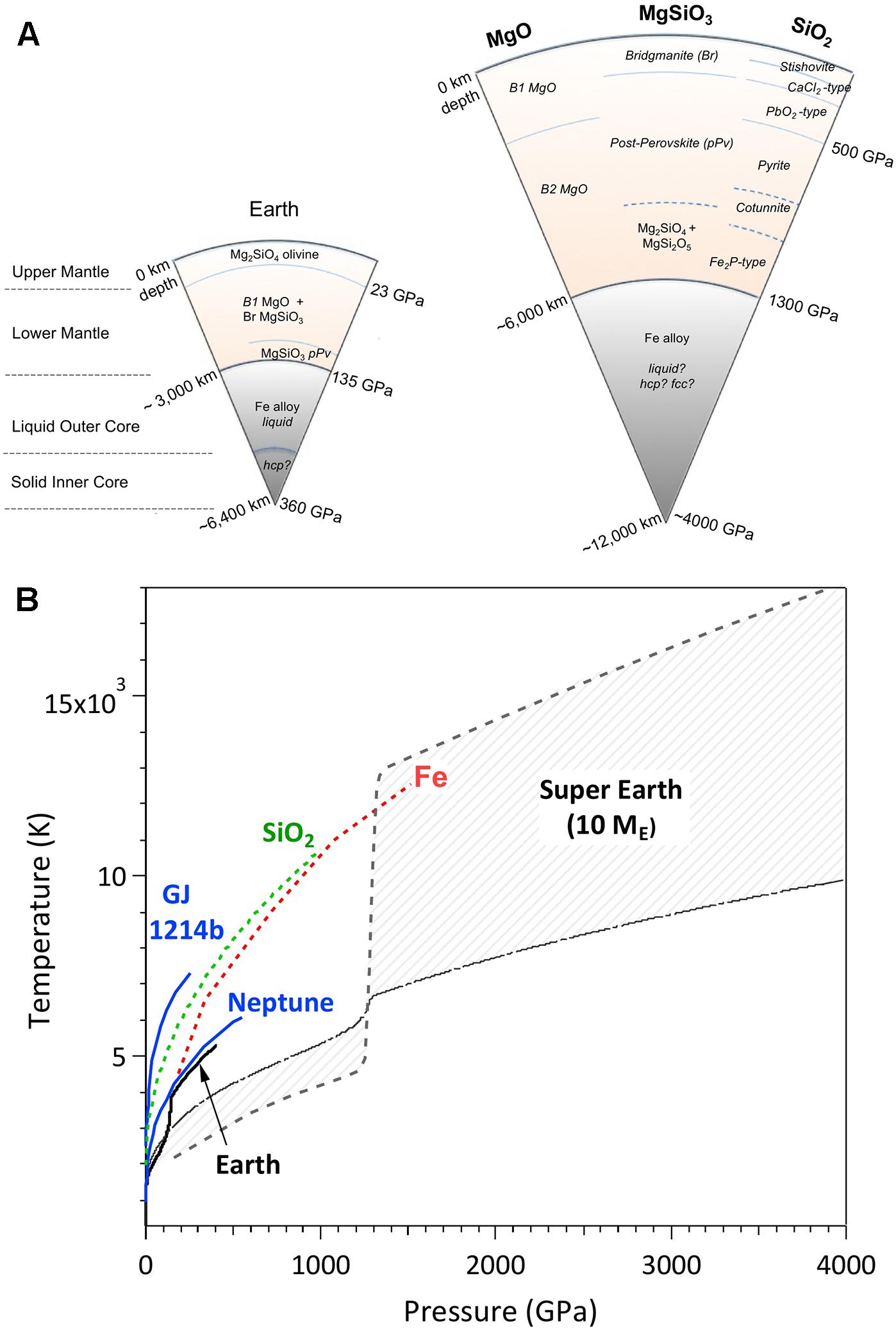
Frontiers Ultra-High Pressure Dynamic Compression of Geological Materials

Compressibility factor `Z=(PV)/(RT)`. Considering ideal gas, real gas, and gases at critical sta
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
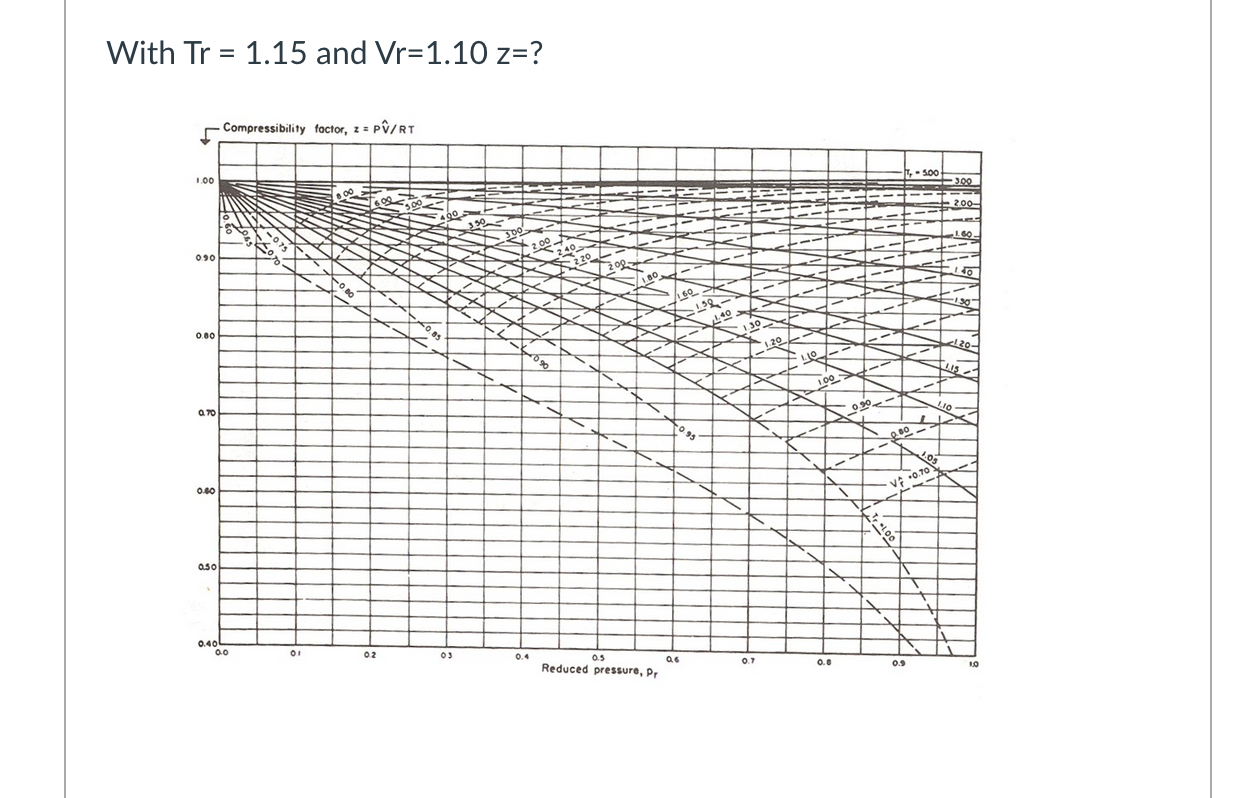
Solved With Tr = 1.15 and Vr=1.10 z=? - Compressibility

PDF) Predicting the compressibility factor of natural gases containing various amounts of CO2 at high temperatures and pressures

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Compressibility factor - Wikipedia

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is