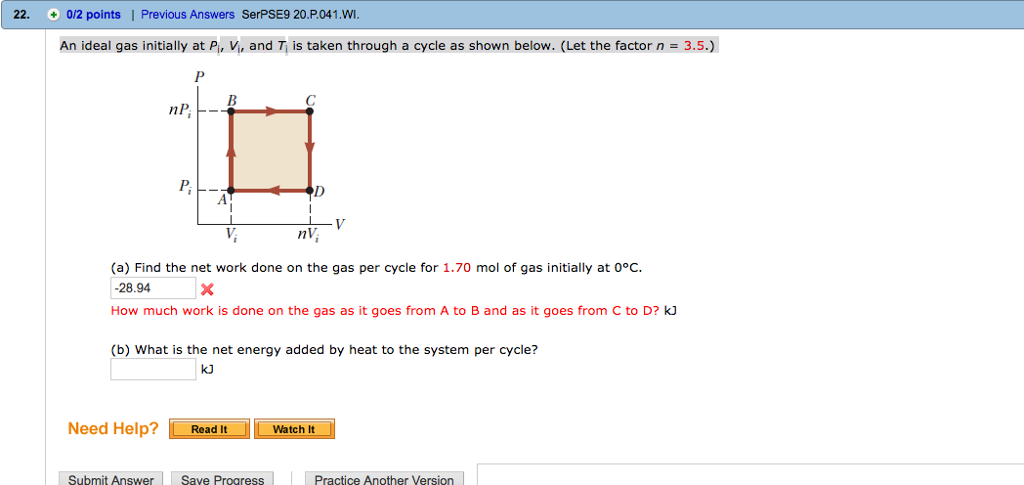
An ideal gas initially P_i ,V_i , and T_i is taken through a cycle
Click here:point_up_2:to get an answer to your question :writing_hand:an ideal gas initially at pi vi and ti is taken through a cycle
Click here👆to get an answer to your question ✍️ An ideal gas initially P-i -V-i - and T-i is taken through a cycle as shown in Figure- -a- Find the net work done on the gas per cycle 1-00 mol of gas initially 0-0C- -b- What is the net energy added by heat to the gas per cycle
Carnot cycle hi-res stock photography and images - Alamy

The figure shows a reversible cycle through which

An ideal gas initially at pressure P0, volume V0, and temperature T0 is taken through the cycle described in Figure P12.54, with n = 4 and m = 3. Figure P12.54 (a)
Joule expansion - Wikipedia

What are PV diagrams? (article)
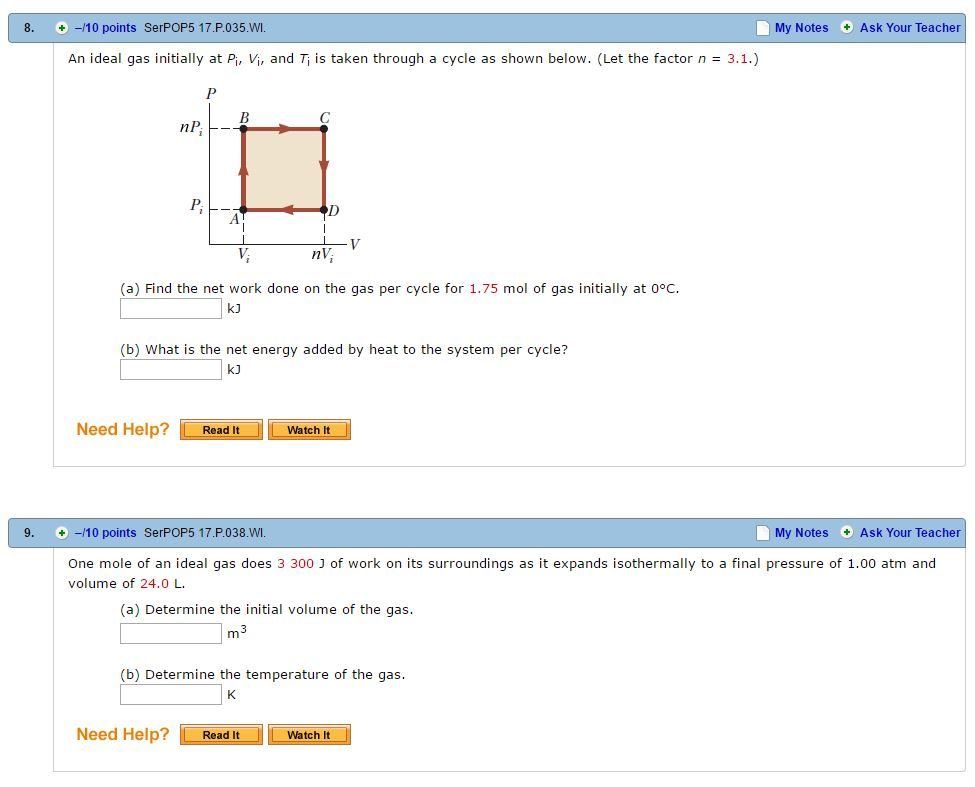
Solved An ideal gas initially at P_i, V_i, and T_i is taken

Thermodynamics problems
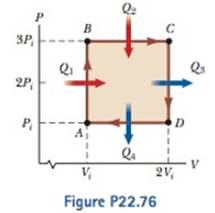
A 1.00-mol sample of a monatomic ideal gas is taken through the cycle shown in Figure P22.76. At point A, the pressure, volume, and temperature are P i , V i
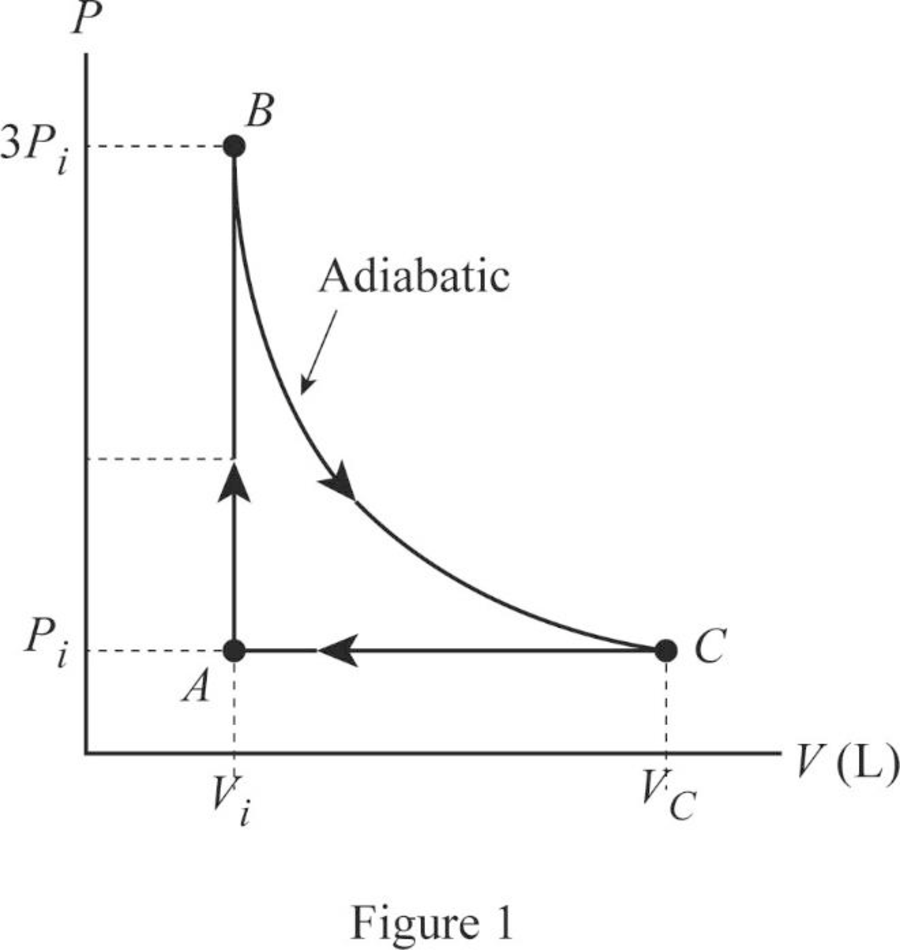
An ideal gas with specific heat ratio γ confined to a cylinder is put through a closed cycle. Initially, the gas is at P i , V i , and T i .

If one mole of an ideal gas at P1,V1,T is allowed to expand reversibly and isothermally A toB its pressure is reduced to 12 of original pressure see figure. This is followed
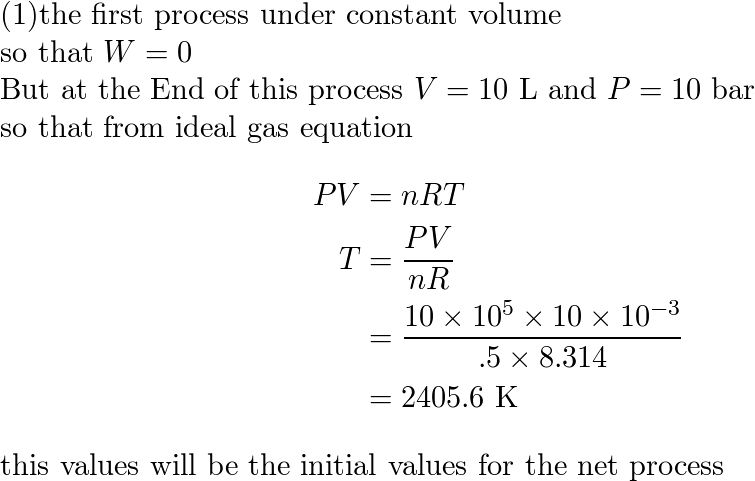

01-Thermodynamic-process-Theory