
At a high pressure, the compressibility factor (Z) of a real gas is us
At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.
Gas Laws – First Year General Chemistry

At a constant pressure, what should be the percentage increase in the
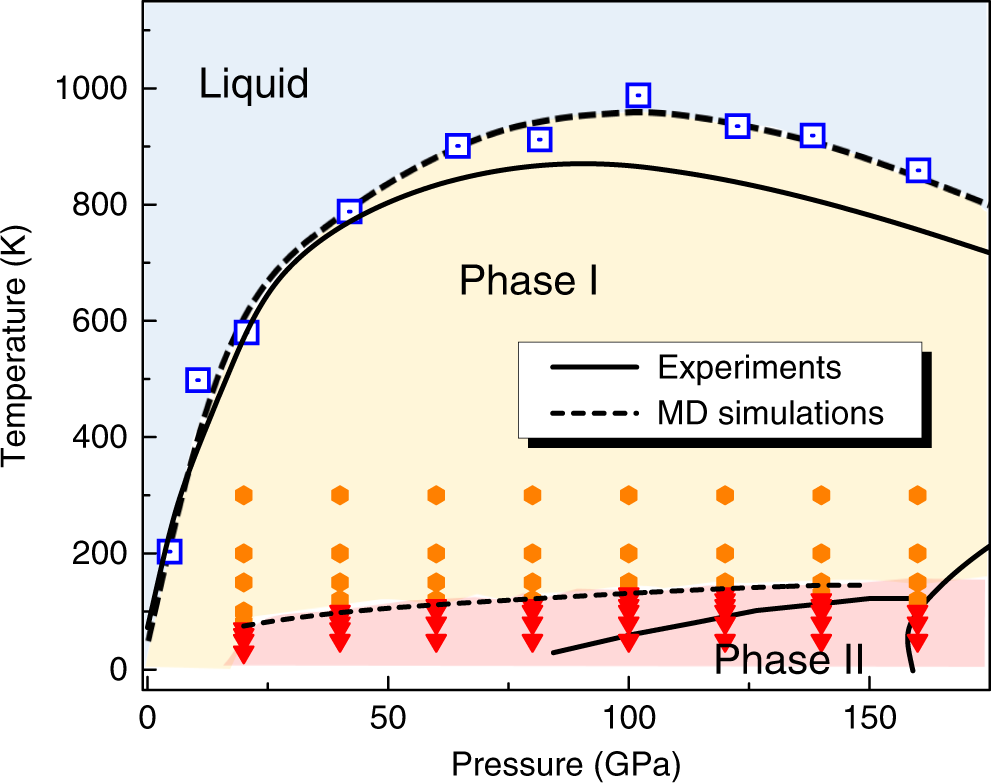
Understanding high pressure molecular hydrogen with a hierarchical machine-learned potential
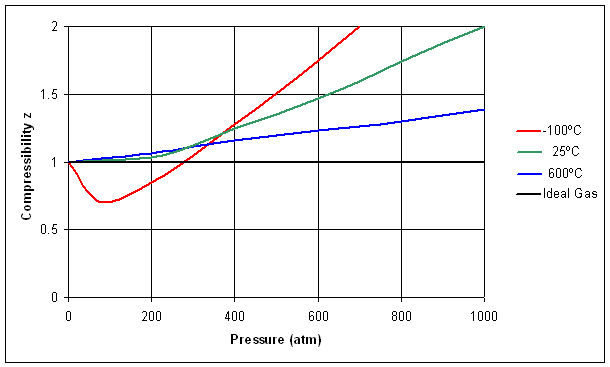
Compressor performance and thermodynamics
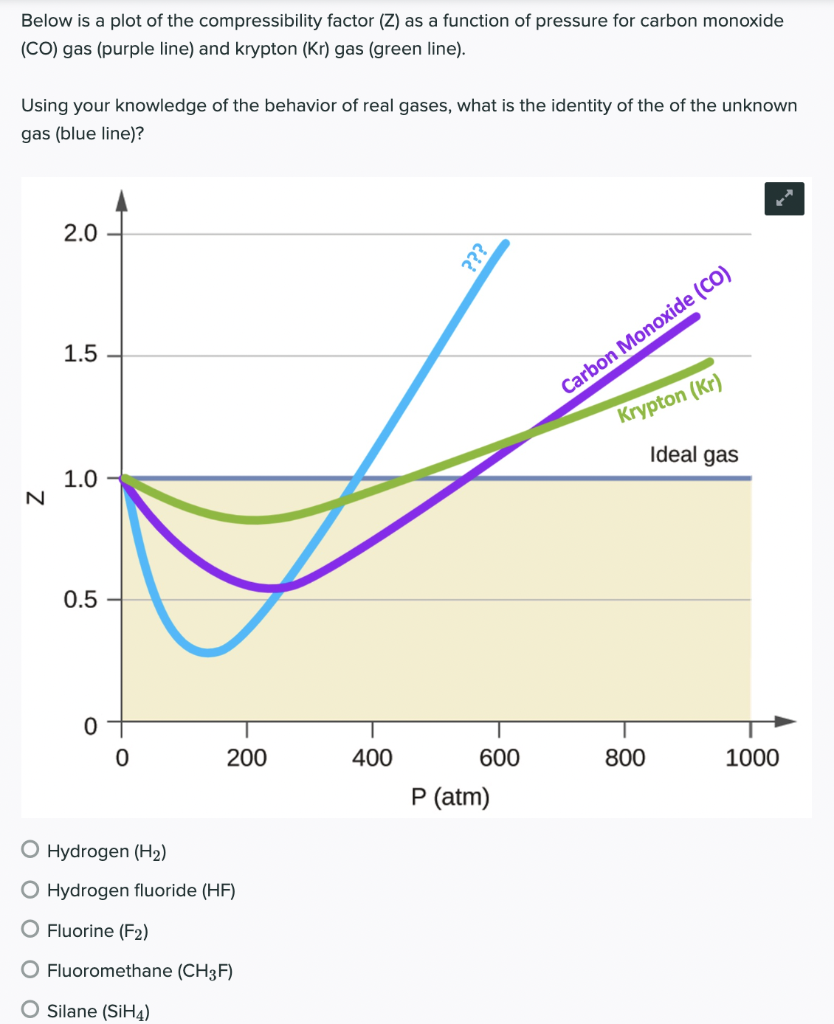
Solved Below is a plot of the compressibility factor (Z) as
How does real gas occupies more volume than an ideal gas at high pressure? - Quora
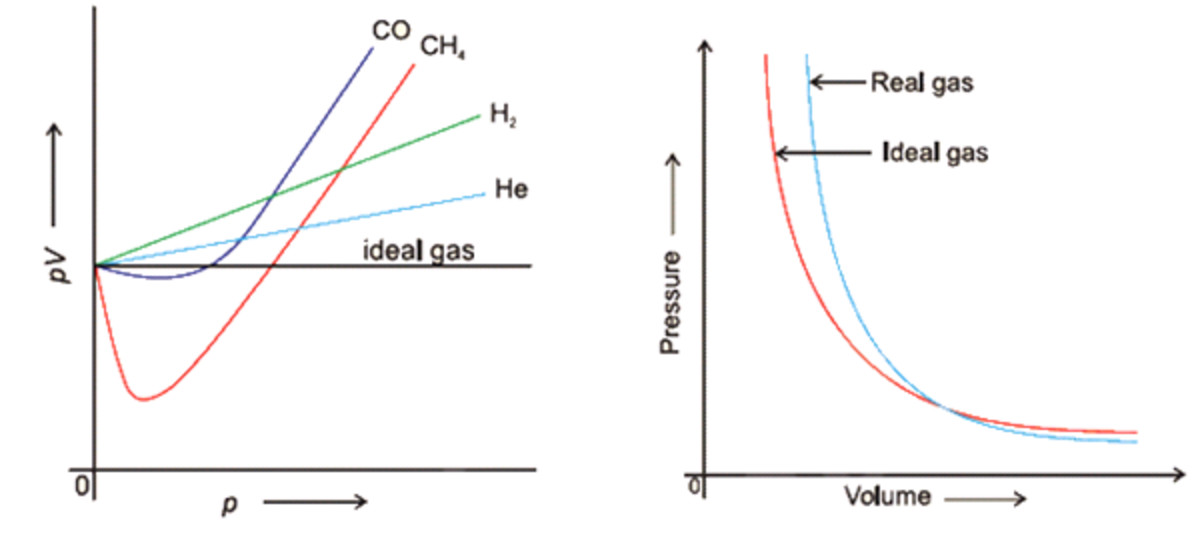
Real Gases - Chemistry, Class 11, States of Matter
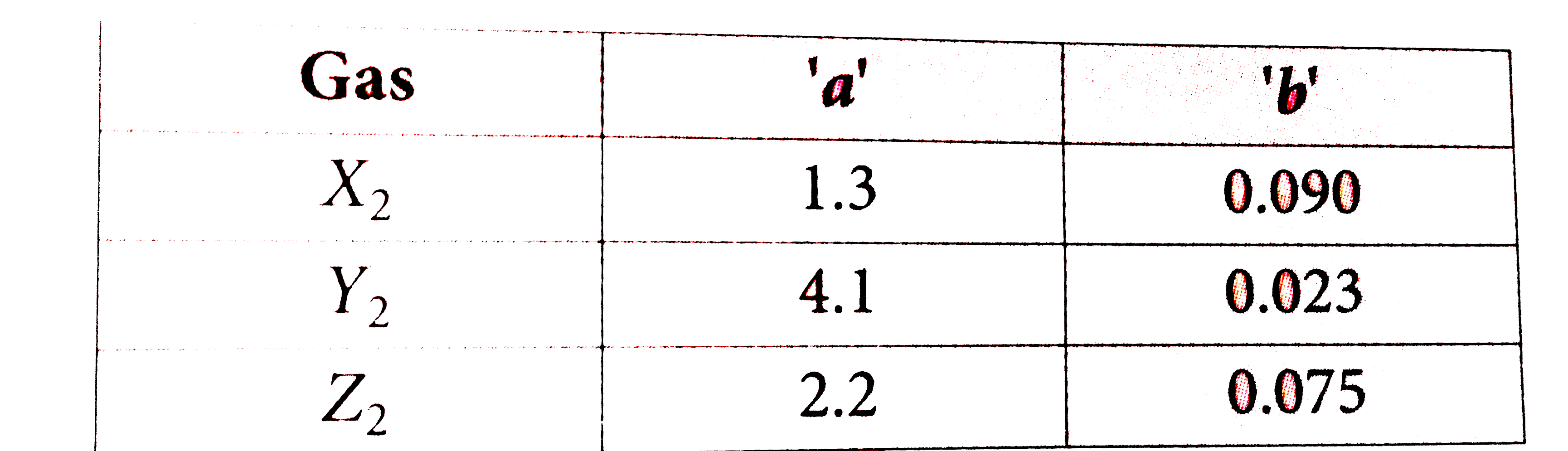
The ratio of Van Der Waal's constants a and b, ((a)/(b)) has the dime
Energies, Free Full-Text

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

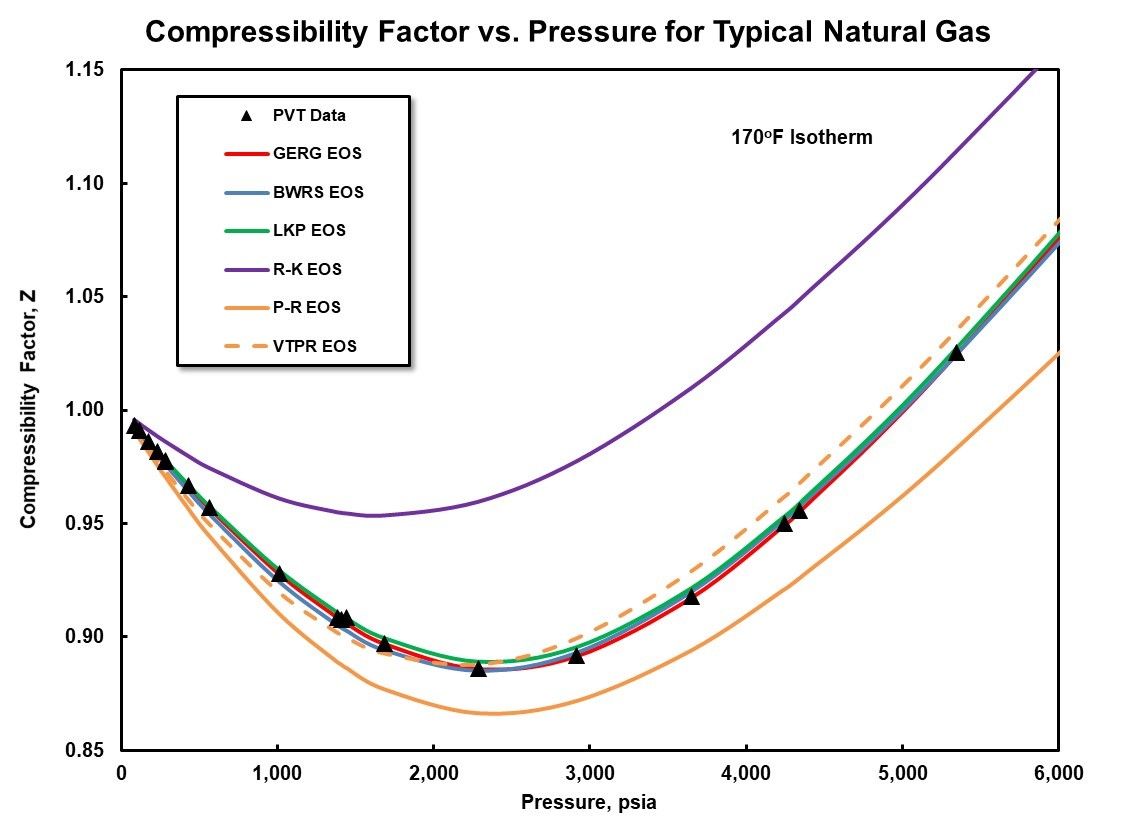
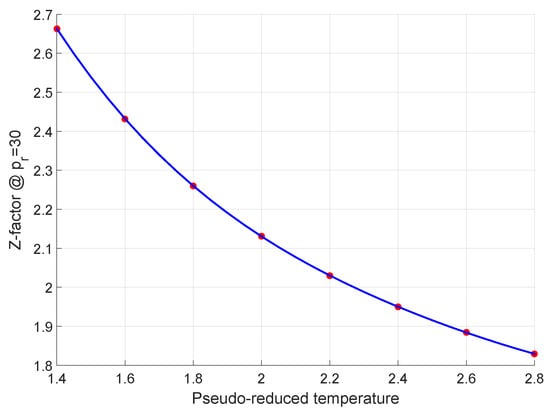
Ch2, Lesson E, Page 9 - Generalized Compressibility Chart
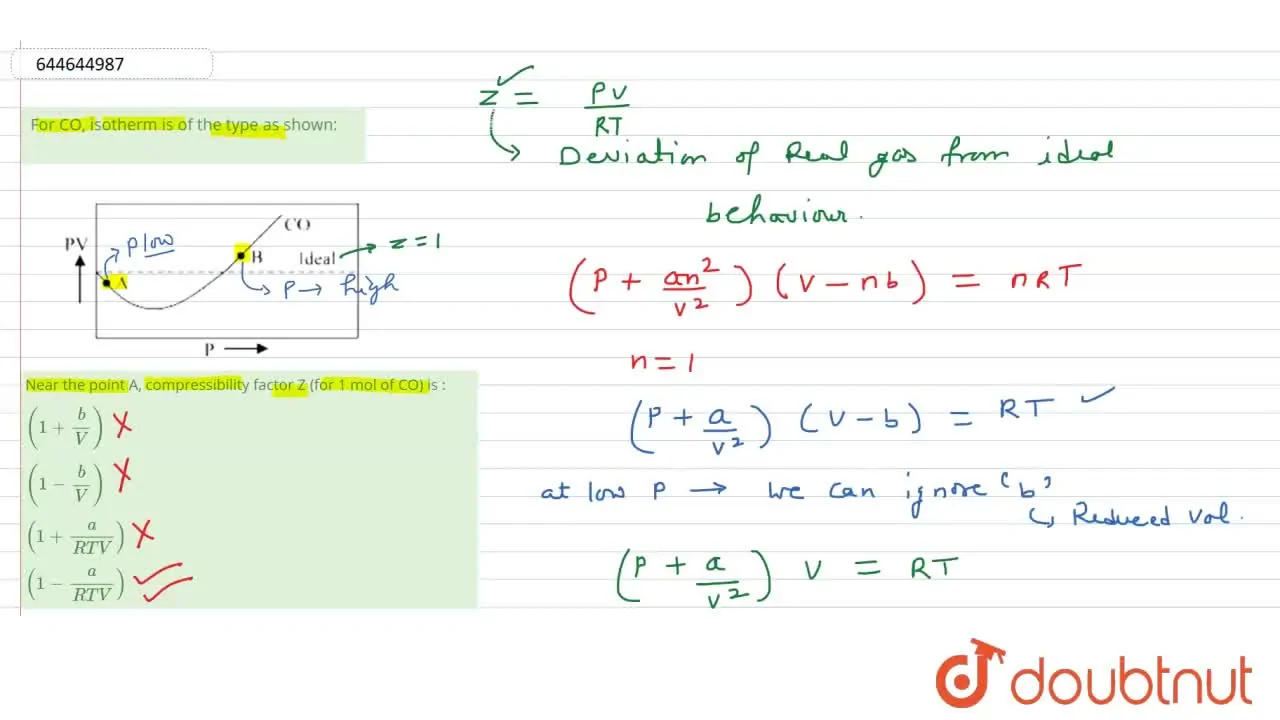
For CO, isotherm is of the type as shown: Near the point A, compr
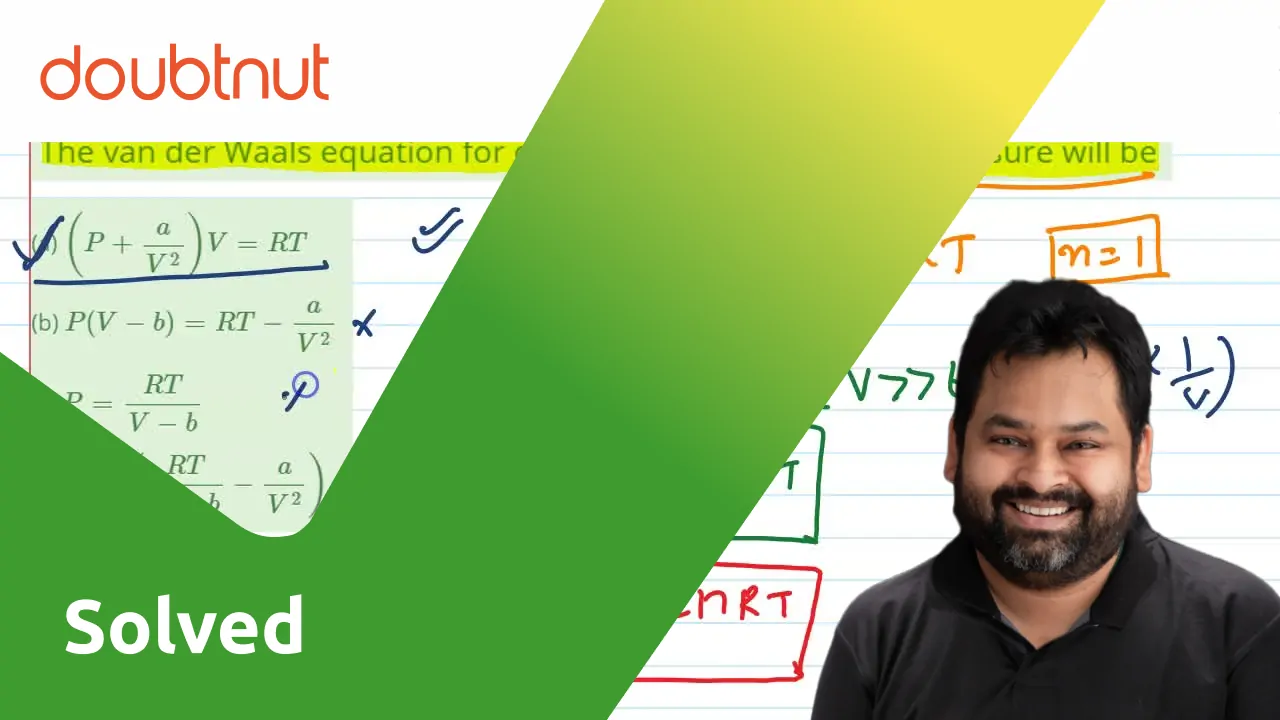
The van der Waals equation for one mol of CO(2) gas at low pressure wi









