32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

Solved Hydrogen reacts with oxygen to form water. This

SOLVED: Which is the limiting reactant when 5.00 g of H2 and 10.0

Q. 88.6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced and the amount of excess reagent left.
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting
How many grams of water can be produced if sufficient hydrogen

3.0 g of H_(2) react with 29.0 g O_(2) to yield H_(2)O (i) What is

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water

Hydrogen production from water: past and present - ScienceDirect

80g of H2 is reacted with 80g of O2 to form water; what are the
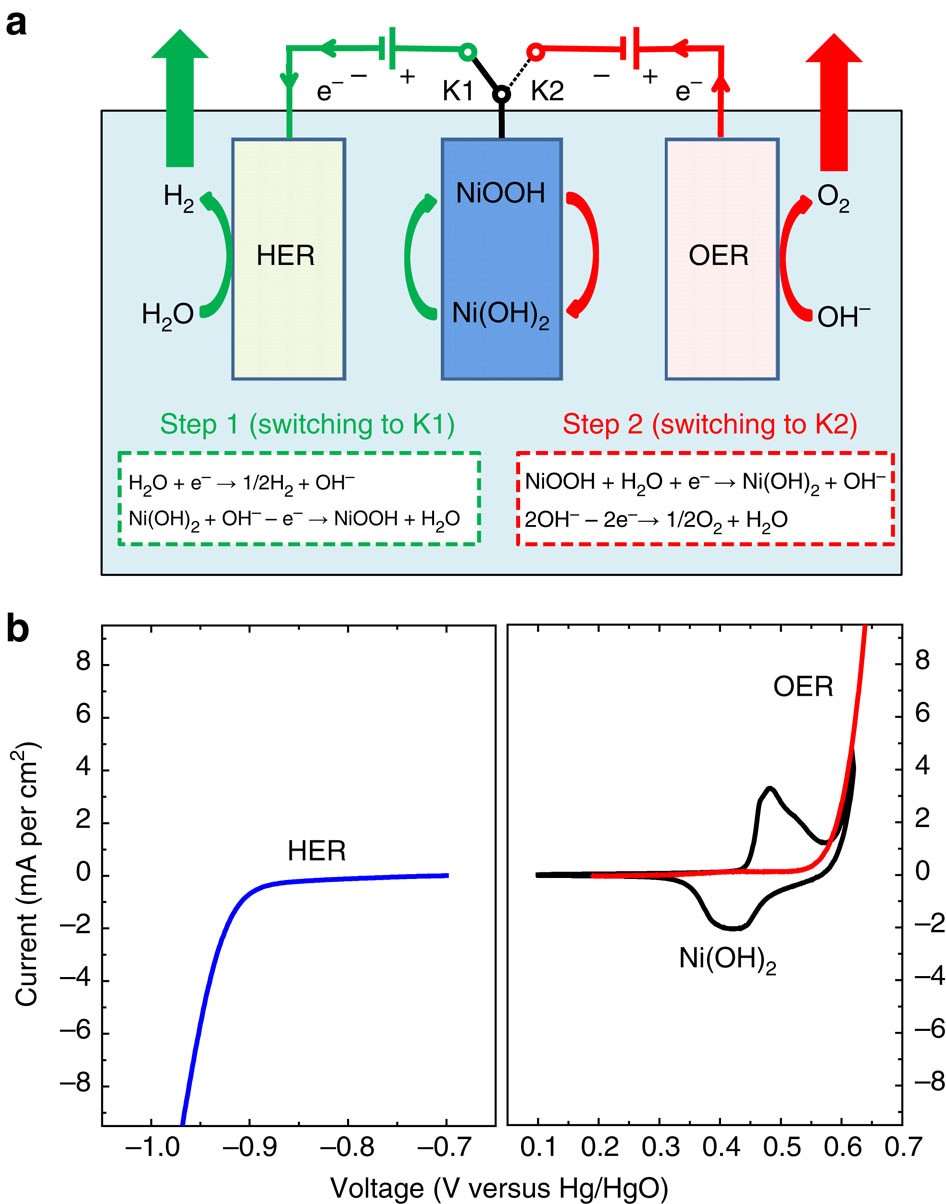
Separating hydrogen and oxygen evolution in alkaline water
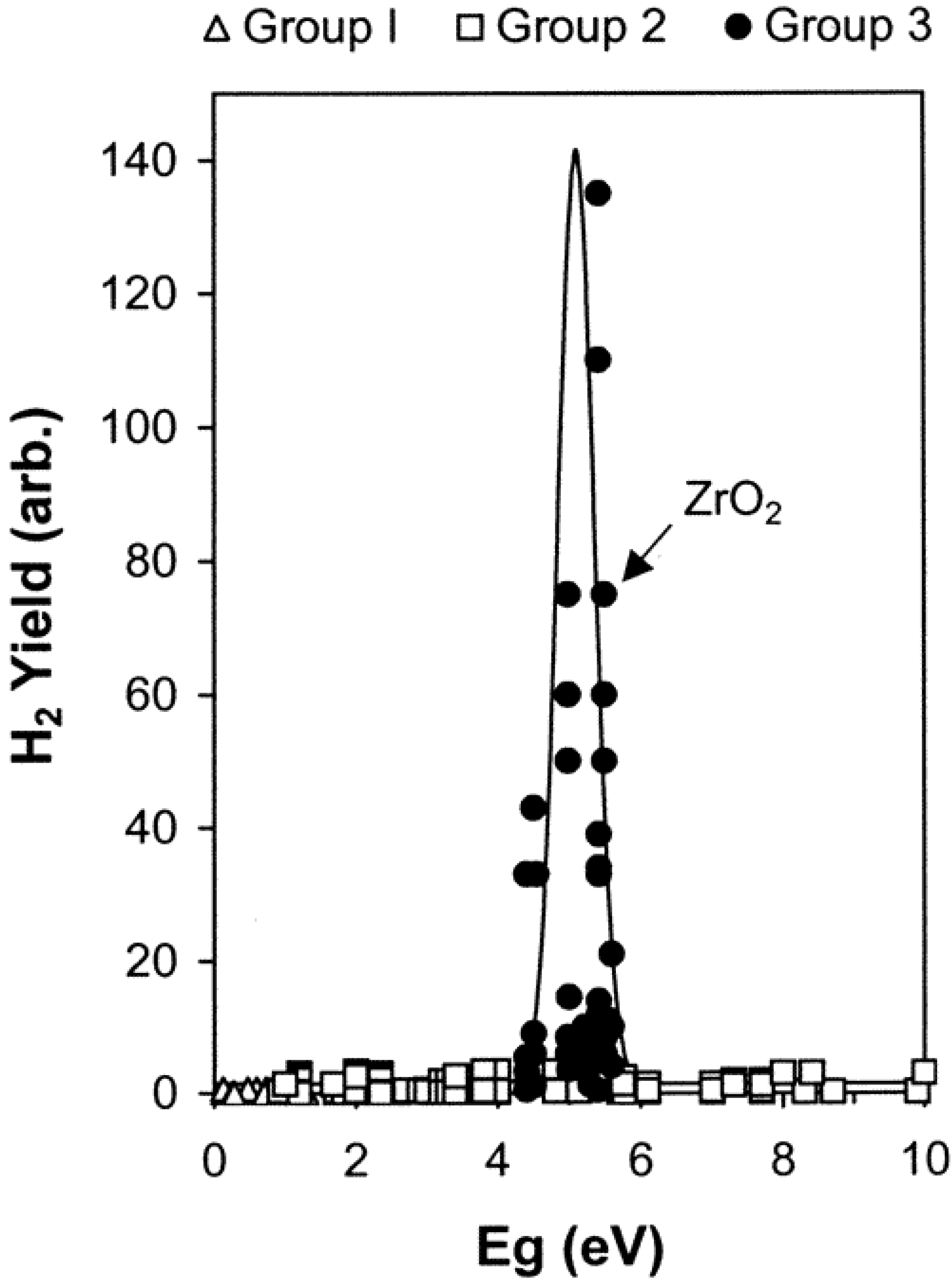
Water, Free Full-Text







