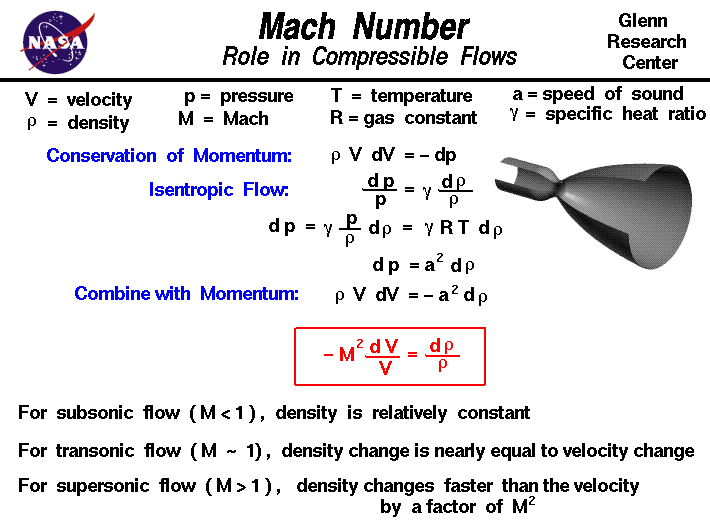
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
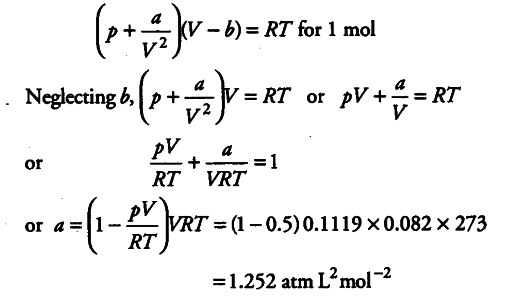
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible

b 26. The compressibility factor 1 mole of a van der Waal's gas Boyle temperature is 1+ VIV-yo) Find the value of x + y. tronarding the van property?

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

A gas has a compressibility factor of 0.5 and a molar volume of 0.4 dm3 mol− 1 at temperature of 800K

Physical Chemistry The Compression Factor (Z) [w/1 example]