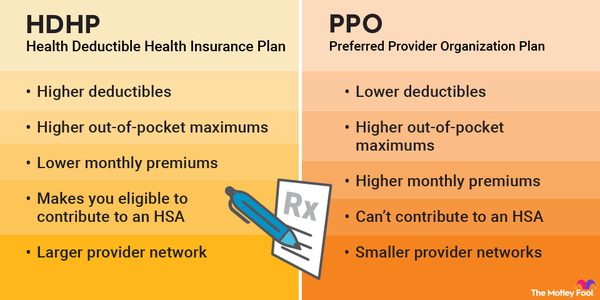
Crucial Steps for Singapore Medical Device Registration & HSA Approval
Discover the crucial steps for successful Singapore medical device registration and HSA approval. Operon Strategist offers expert guidance, classification insights, and comprehensive support. Contact Operon Strategist to learn more and navigate the regulatory landscape with confidence.
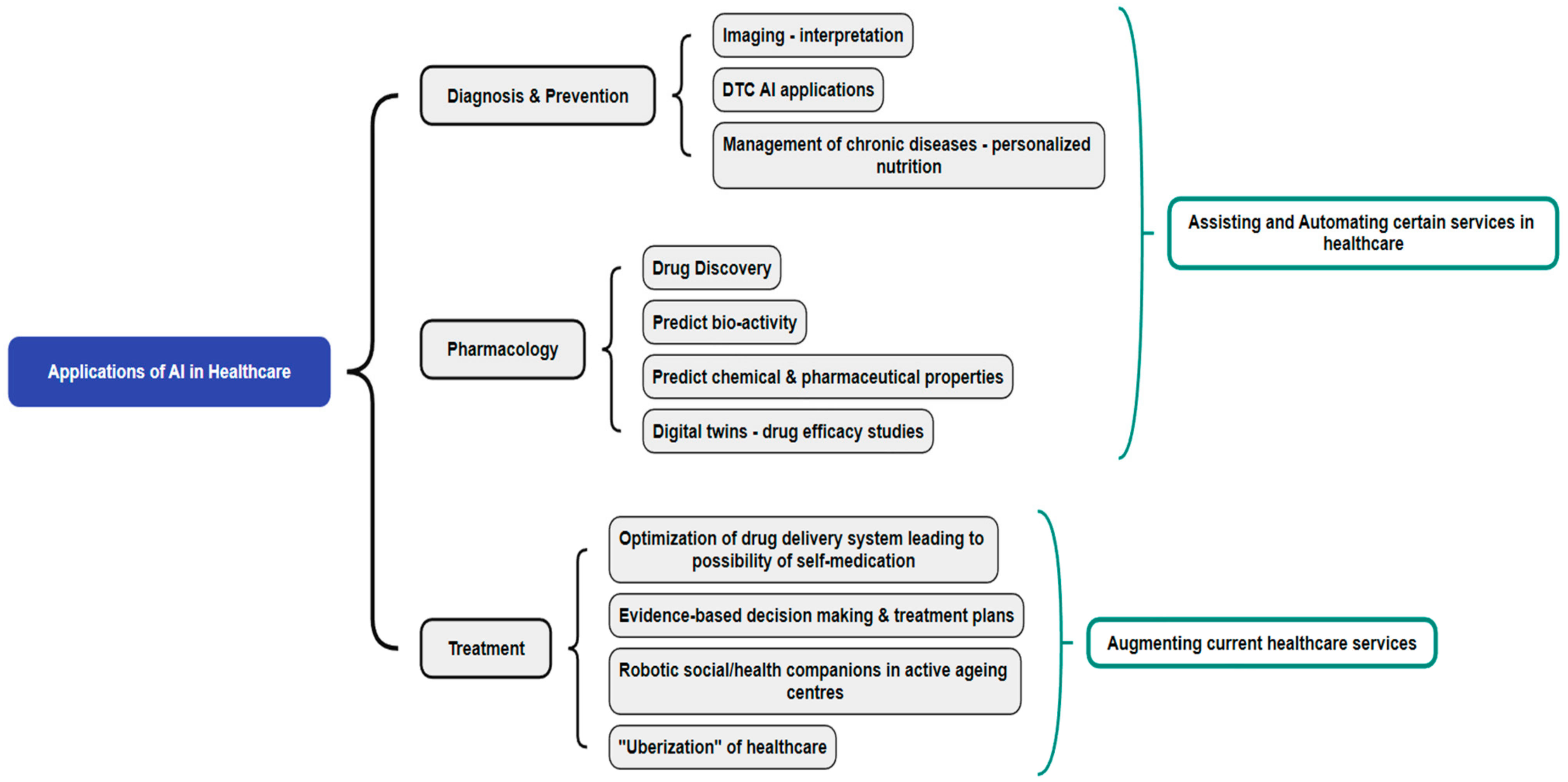
Healthcare, Free Full-Text

Academic Report on Singapore HSA Class D and Australia TGA Class III Medical Device Regulatory Overview & Strategy

Singapore's HSA - Global Regulatory Partners, Inc.

HSA Revised Guidance on Medical Device Product Registration: Overview
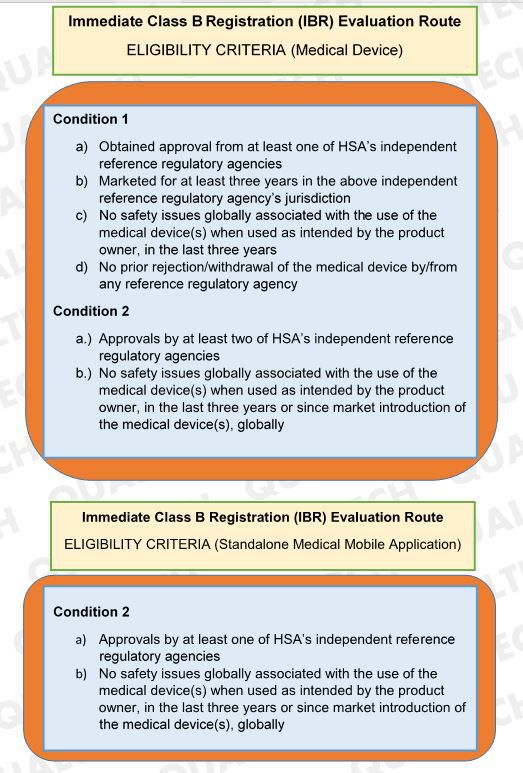
SINGAPORE: HSA Improves Criteria For Immediate Class B Registration (IBR) Evaluation Route – April, 2019
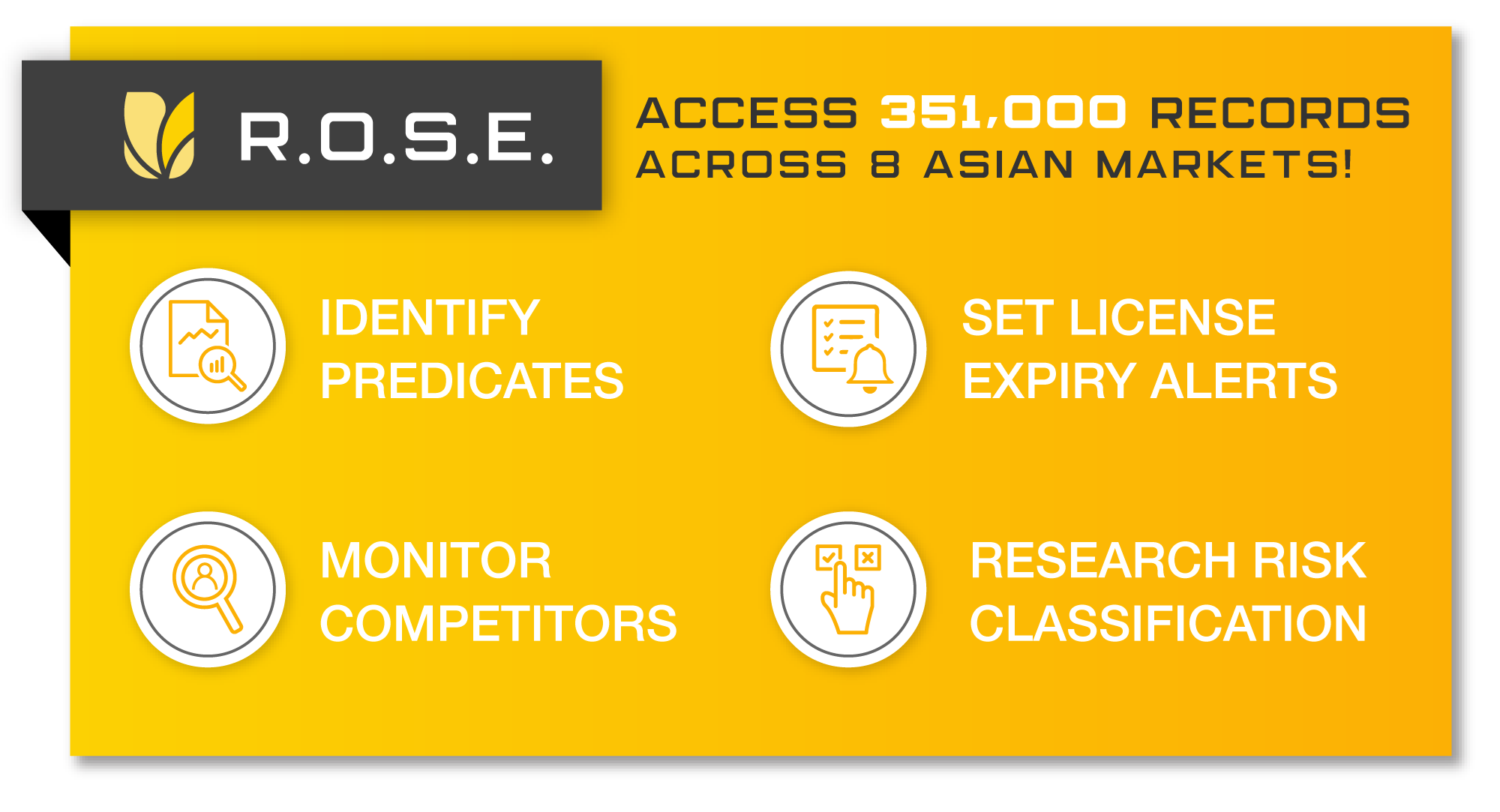
Identifying Predicate Devices in Asian Countries

The Singapore Guidance on Software Medical Devices
Novavax gets Singapore's HSA approval for prototype Nuvaxovid

Understanding the Medical Device Registration Process in Singapore

Singapore's HSA - Global Regulatory Partners, Inc.
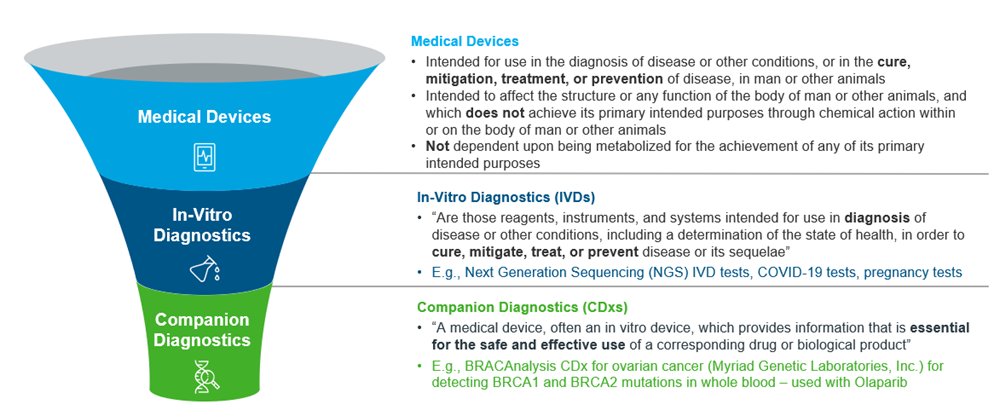
Overview of Companion Diagnostics and its Regulatory Trends in Asia Pacific - IQVIA