
Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.

Applications for Medical Device Investigational Testing Authorizations Guidance Document

Essential Documents Required for Conducting Clinical Trials
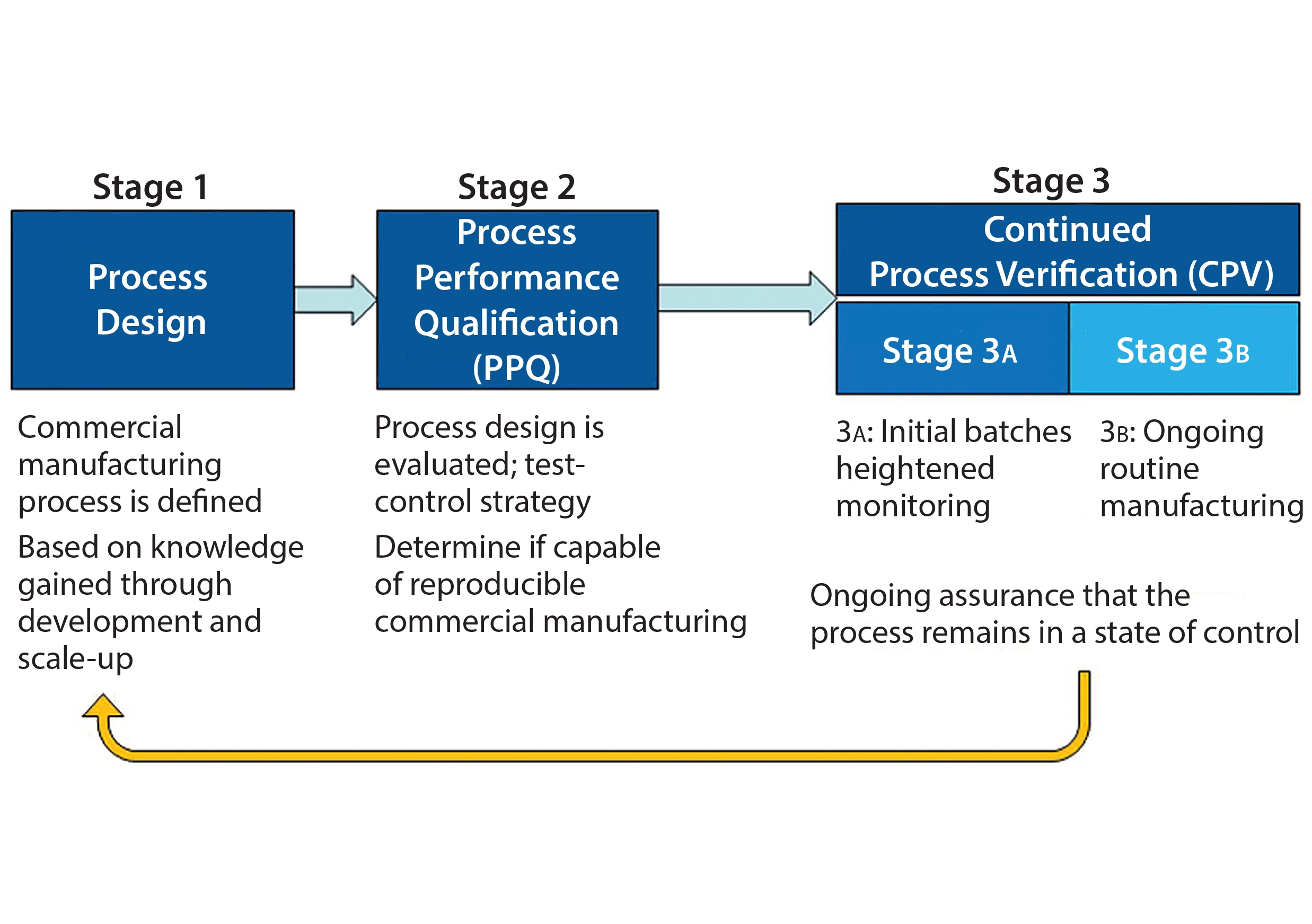
Continued Process Verification: Evolution of Biopharmaceutical Control Strategy - CMC Forum

ESMO Guidance for Reporting Oncology real-World evidence (GROW) - ESMO Real World Data and Digital Oncology

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Guidance on how to complete the application for a new medical device licence: Overview
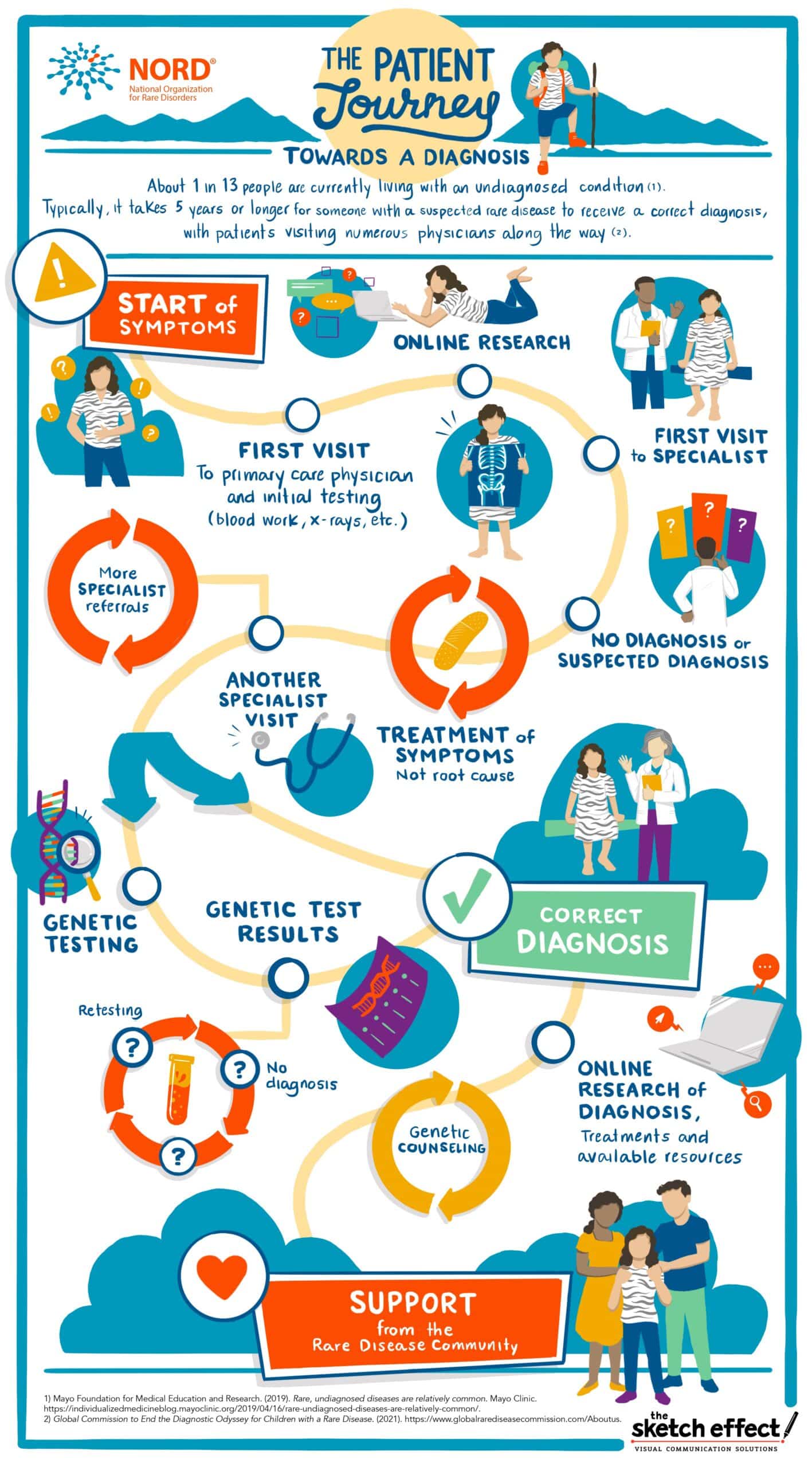
New Patient Journey Infographic Gives A Glimpse Into The Diagnostic Odyssey - National Organization for Rare Disorders

HIPAA Privacy Rule - Updated for 2024

Regulation of “Biomaterials” and Medical Devices

Sustainability, Free Full-Text









