Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases

Liquid 116. Which of the following is the only incorrect statement - Ideal gas X L (1) For the gas (A): 0 and it varies linearly with P Hut2) For the gas (
GitHub - aegis4048/GasCompressibility-py: Gas compressibility z-factor calculator package in Python

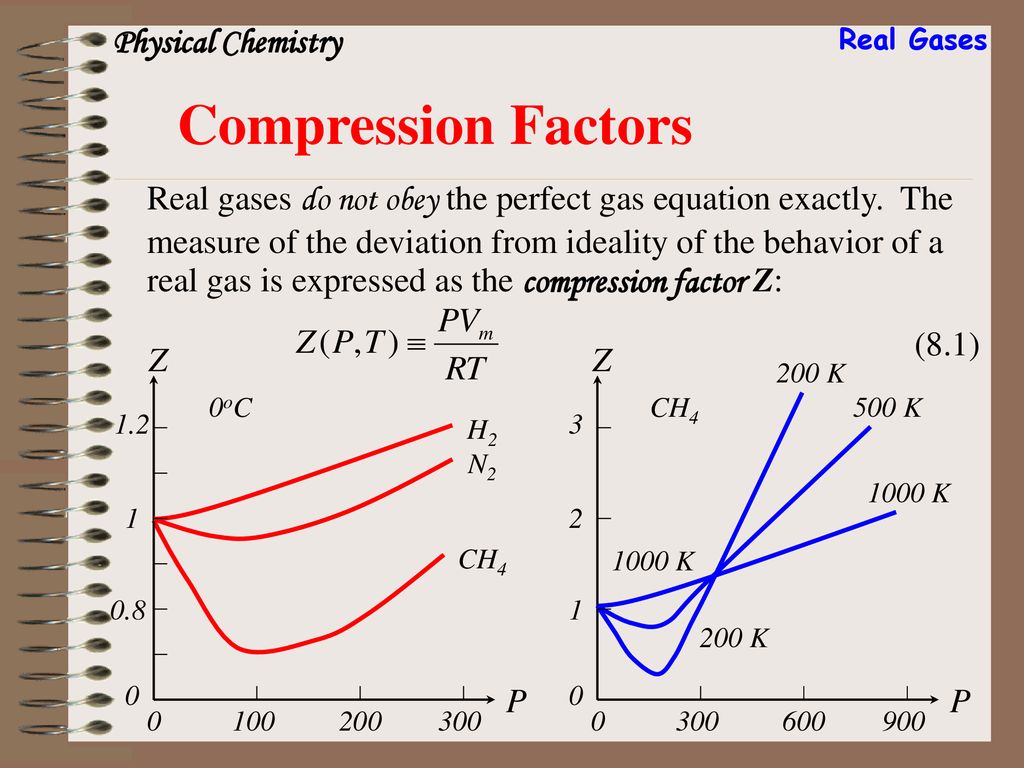
Compressibility Factor Z

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Solved 2. (20 points) At low pressures, the compressibility

English Edition (6 MB pdf) - Saudi Aramco

where Z is the compressibility factor that

Select incorrect statement : (a) we can condense vapour simply by applying pressure (b) to liquef

Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
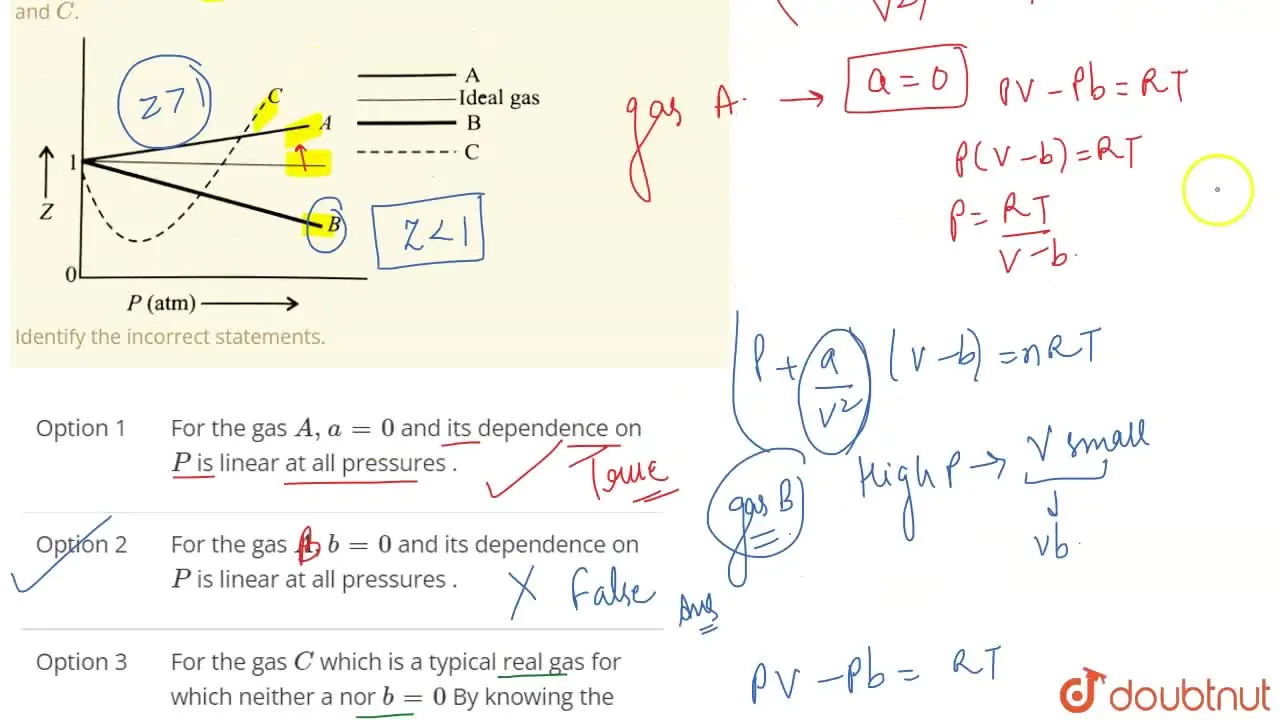
For the gas C which is a typical real gas for which neither a nor b =0

Knjiga, PDF, Fluid Dynamics

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.
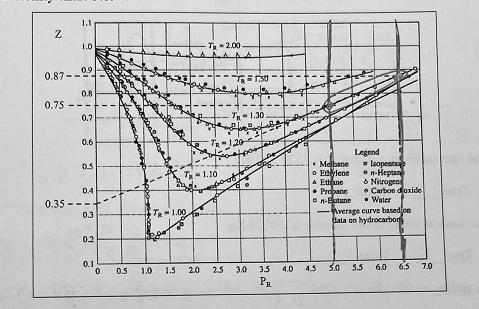
Solved 4. Consider m=1Kg of nitrogen (N2) gas being

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor