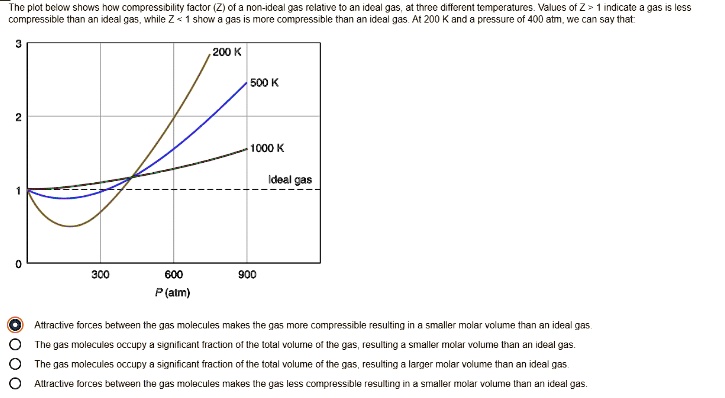
physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In
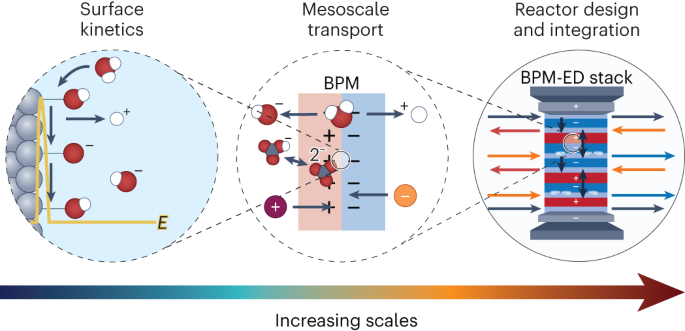
Multi-scale physics of bipolar membranes in electrochemical

Non-Newtonian Flow to the Theoretical Strength of Glasses via
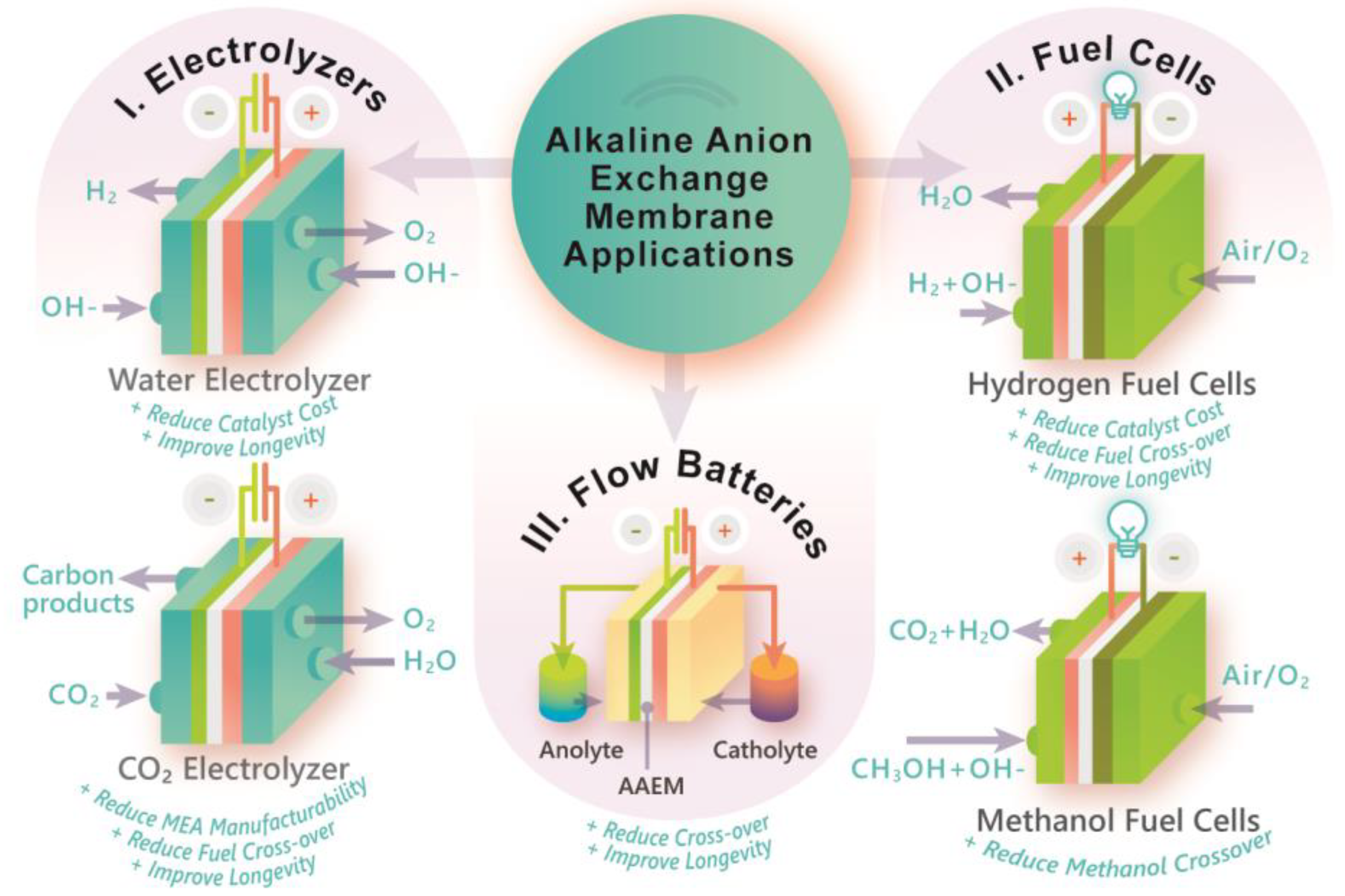
Polymers, Free Full-Text

Which gases have compressibility factor greater than 1 at low
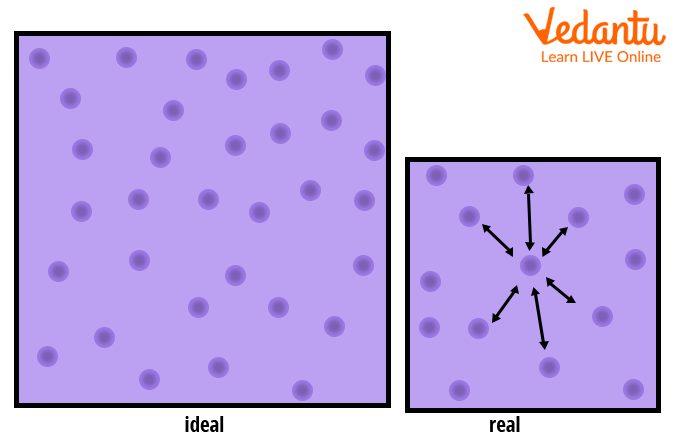
JEE - Compressibility Factor Important Concepts and Tips
Ideal gas - Wikipedia
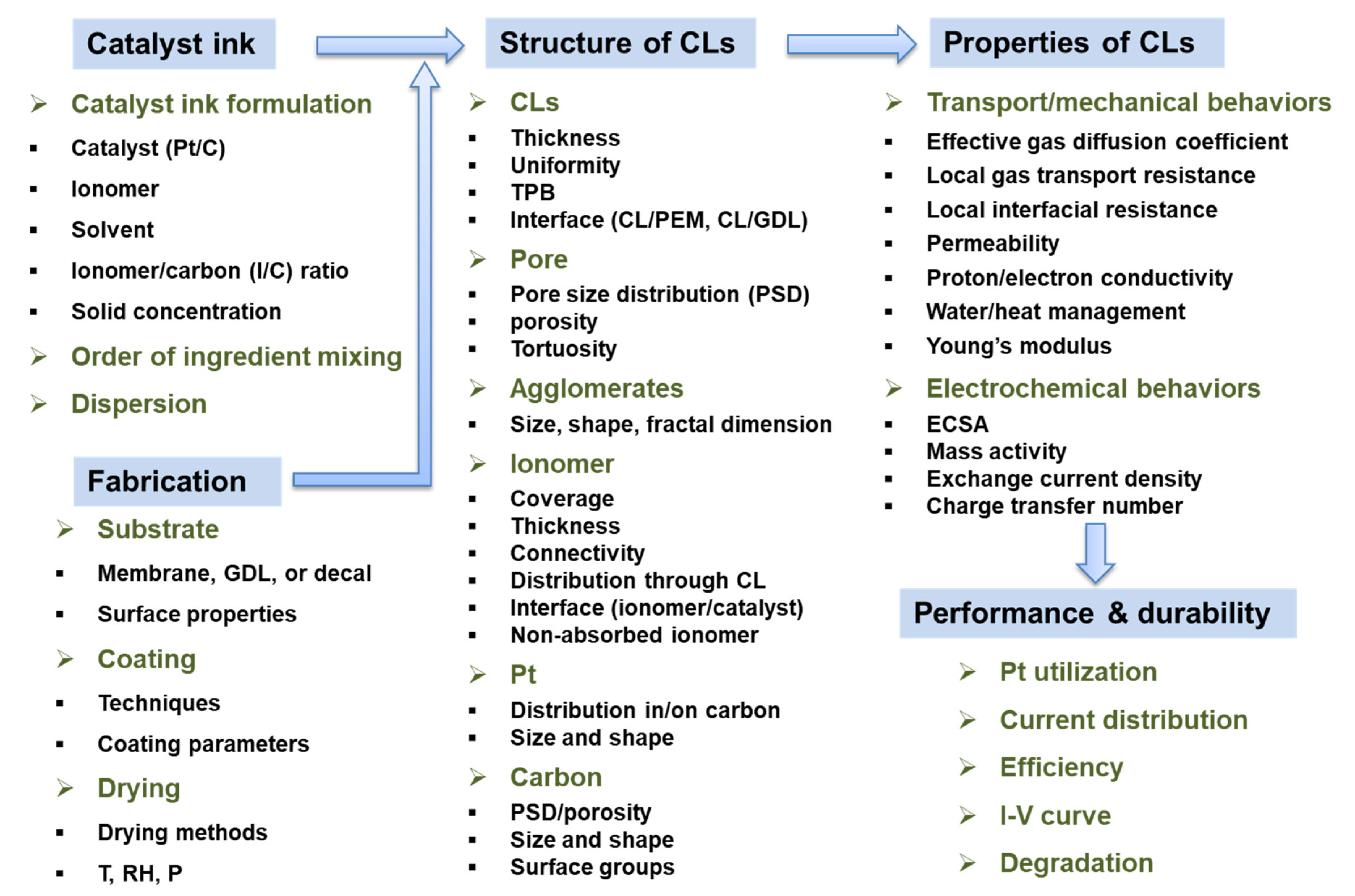
Applied Sciences, Free Full-Text
Temperature-pressure phase diagram for the TMTTF and TMTSF charge
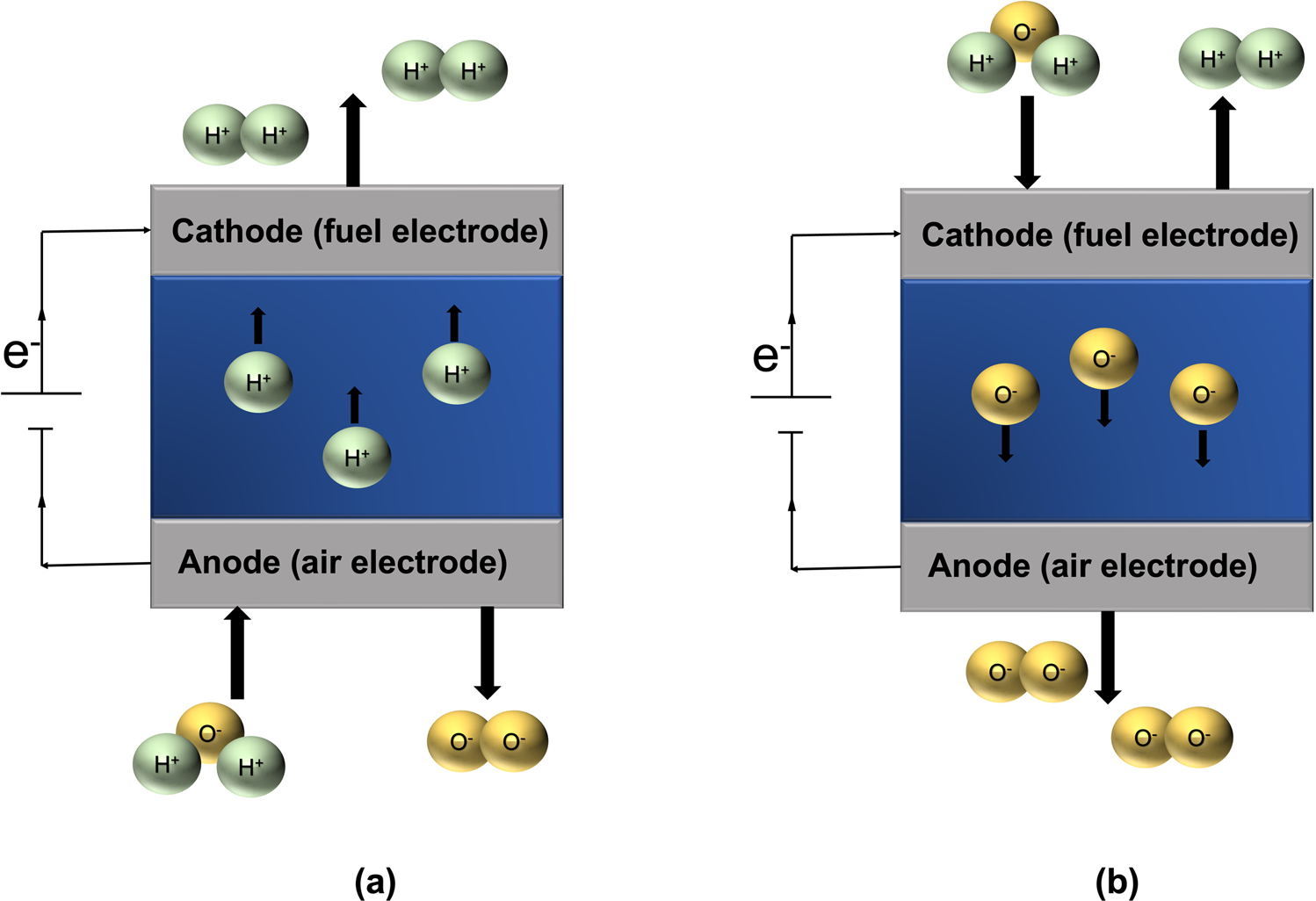
Enhancing the Faradaic efficiency of solid oxide electrolysis
What does a compressibility factor >1 signify, apart from a
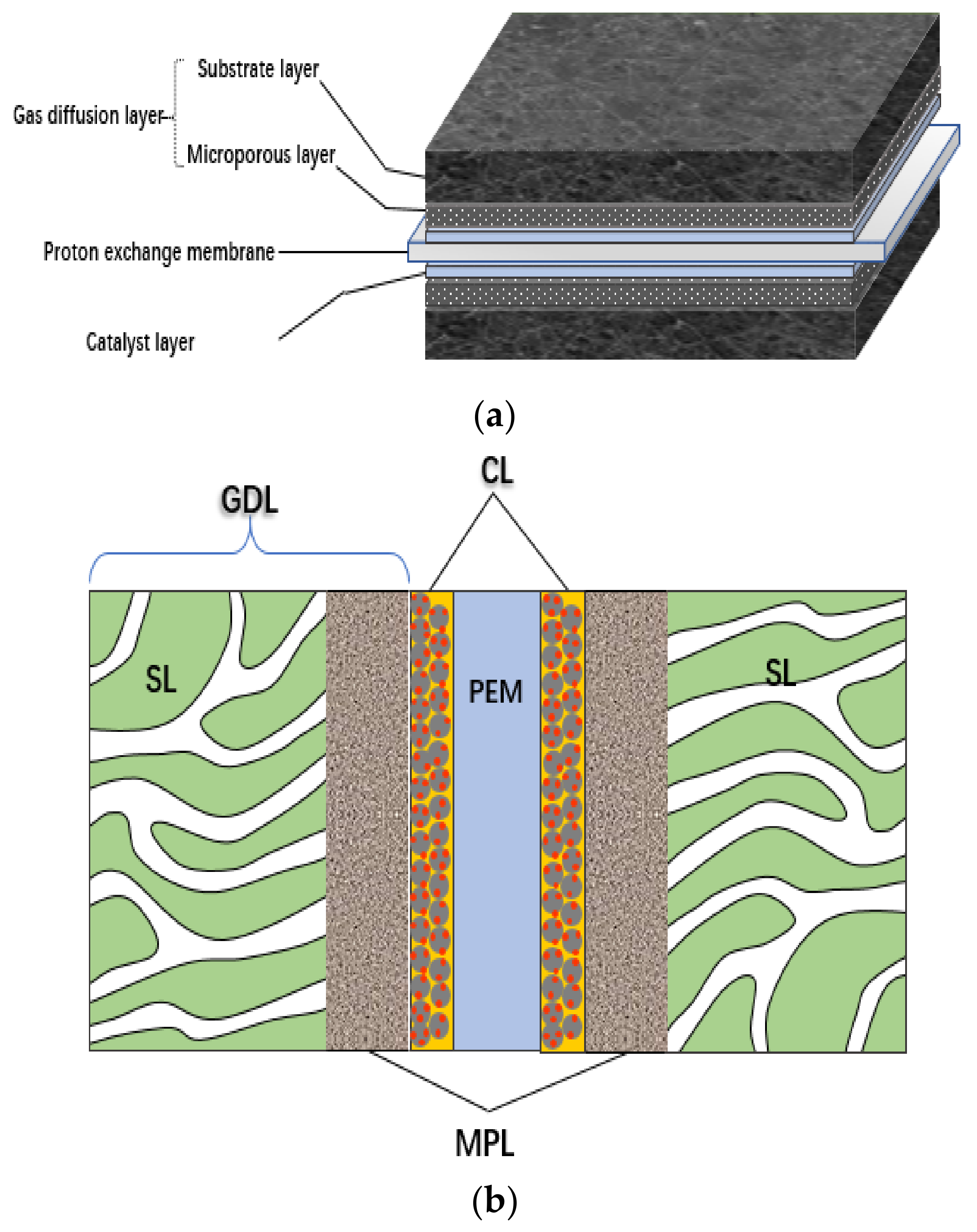
Membranes, Free Full-Text

1.7: Connecting the van der Waals and the viral equations- the

Applied Sciences, Free Full-Text
Effect of pulse-current-based protocols on the lithium dendrite