
The compressibility factor Z for an ideal gas will be
The compressibility factor Z for an ideal gas will be

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

For an ideal gas, the value of compressibility factor `Z(=(pVm)/(RT))` is

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility Factor - Thermodynamics I, EGN 3343, Study notes Thermodynamics
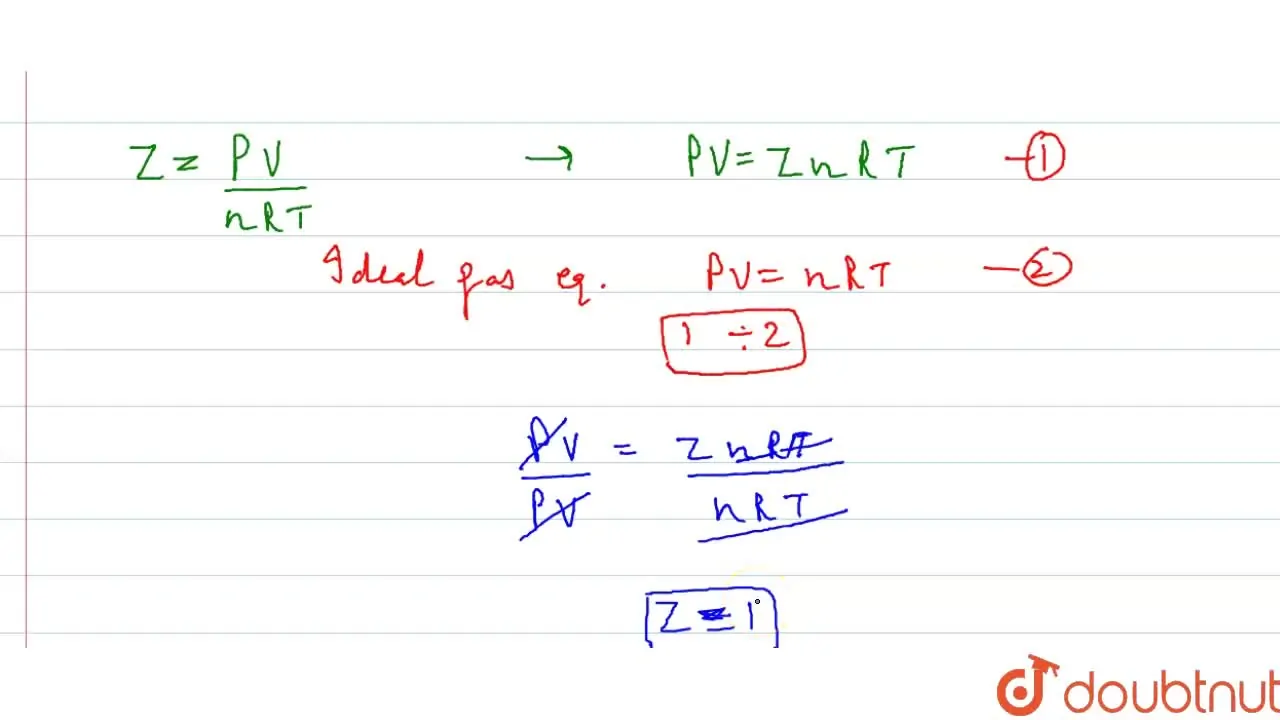
Punjabi] (True/False) The compressibility factor (z) for ideal gases

The van't Hoff factor for aqueous solution of CuSO(4).5H(2)O will be..

Solved 4. Real gas effects can be expressed as departures
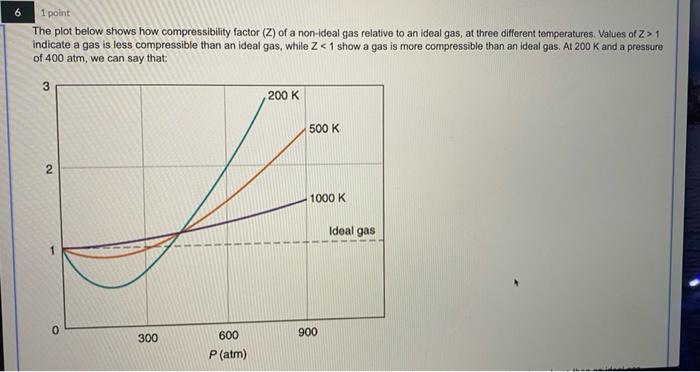
Solved 6 1 point The plot below shows how compressibility

Question No 5 4 Digit Integer Type Question Q.1 to Q.6 are

The value of compressibility factor (`Z`) for an ideal gas is

For a non-ideal gas, the compressibility factor (Z) is defined as: Z









