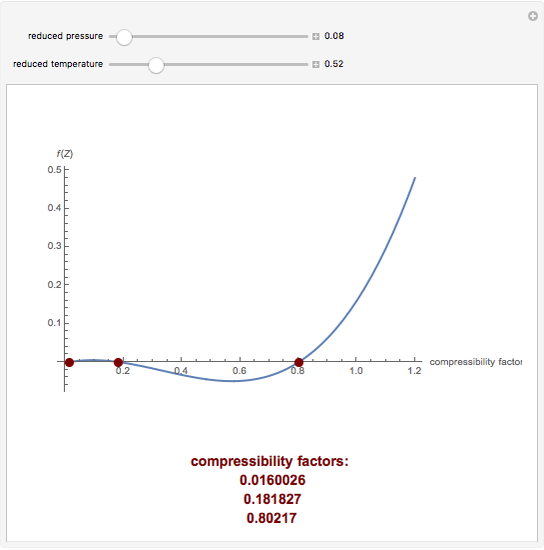
Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule
Why can't anymore atoms enter in the excluded volume (region of 2 atoms) in the volume correction given by van der Waals equation? - Quora
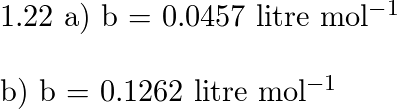
a) A certain gas obeys the van der Waals equation with $a =

Solved 2. (20 points) At low pressures, the compressibility

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0ºC and 100 atm pressure is - Sarthaks eConnect

Welcome to Chem Zipper.com: THE STATE OF MATTER

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
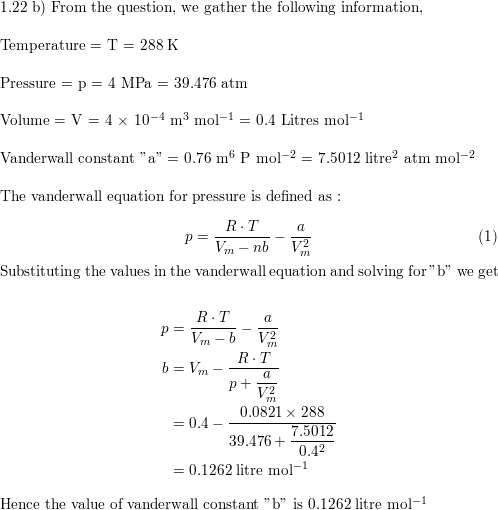
a) A certain gas obeys the van der Waals equation with $a =
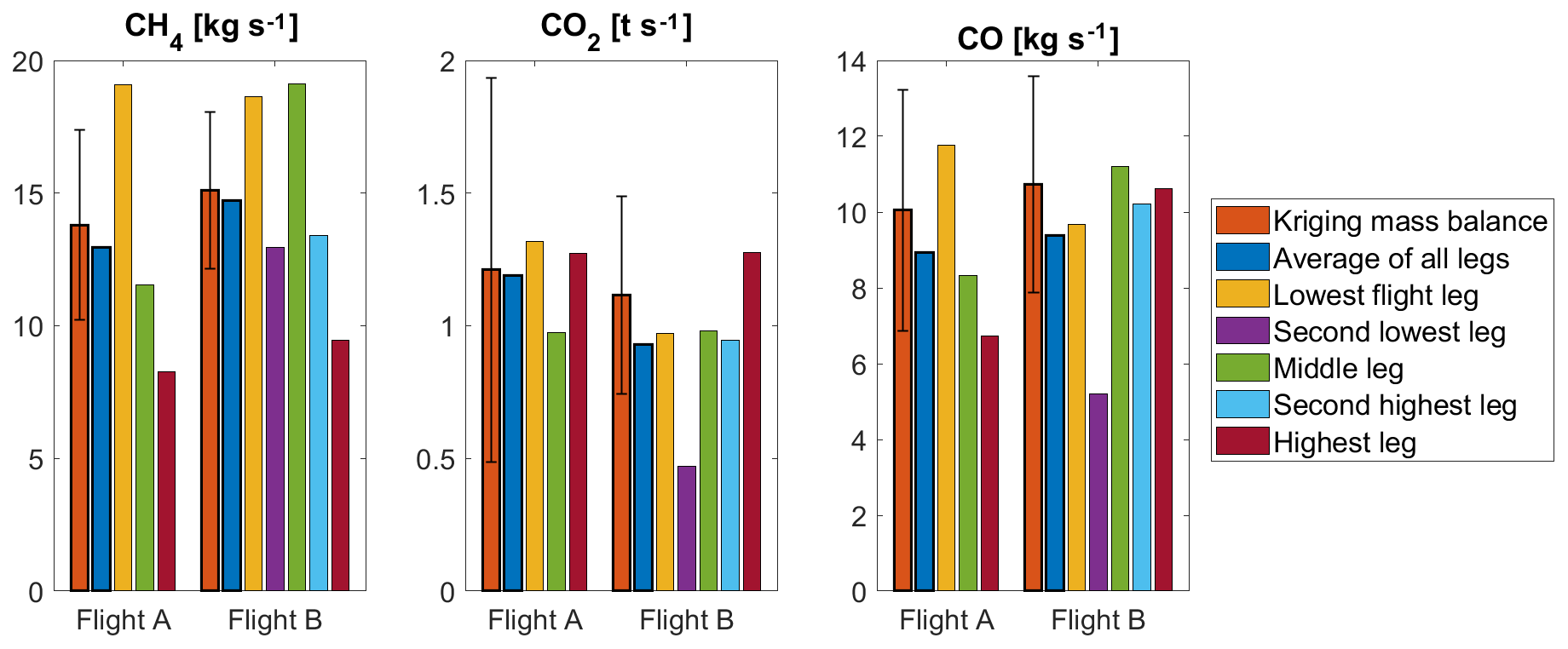
ACP - Estimating CH4, CO2 and CO emissions from coal mining and industrial activities in the Upper Silesian Coal Basin using an aircraft-based mass balance approach
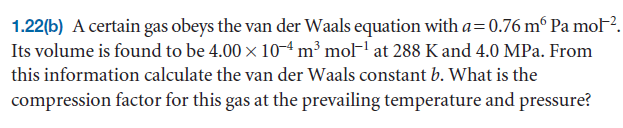
Solved 1.22(b) A certain gas obeys the van der Waals
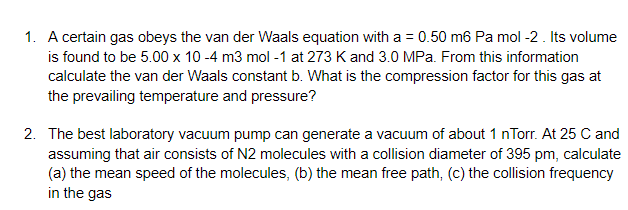
Solved A certain gas obeys the van der Waals equation with a