200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

6.5 g of an impure sample of limestone liberates 2.2 g of CO2 on strong heating. The percentage purity of
When a limestone of mass 150g was heated until it decomposed to CaO, only 63g of CaO were obtained. What is the percentage purity of the limestone? - Quora
Solved] A limestone analyzes CaCO3 92.89 lb MgCO3 5.41 lb Insoluble 1.70 lb
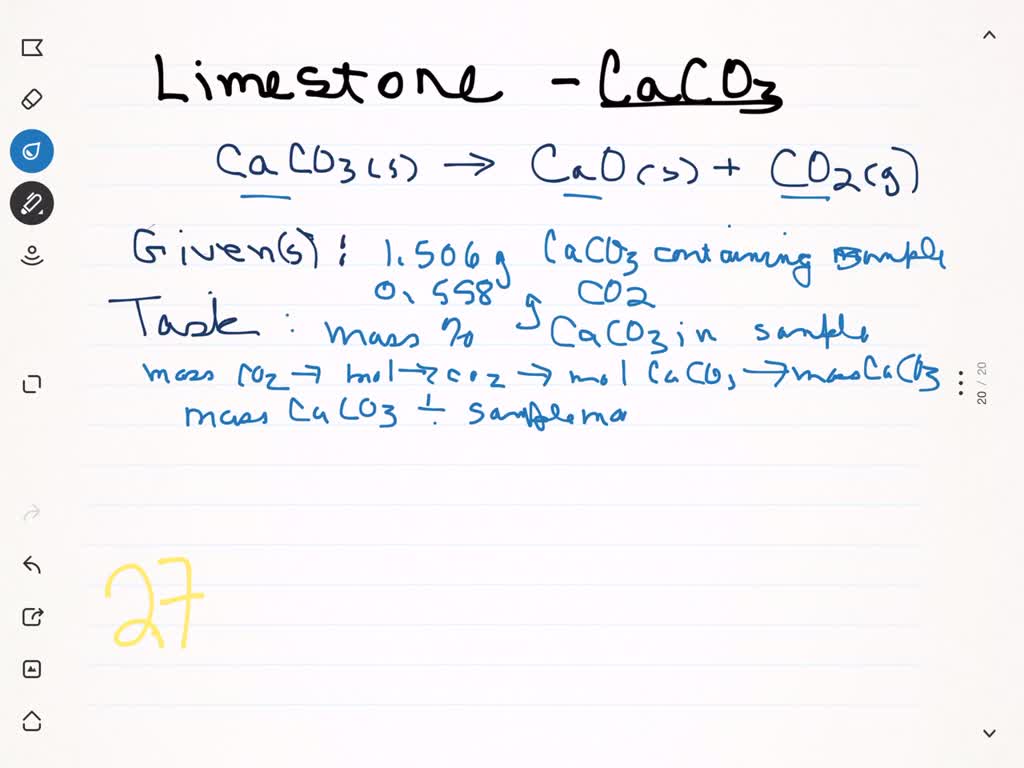
⏩SOLVED:A sample of limestone and other soil materials was heated,…
What quantity of commercial limestone containing 80% CaCO3 on heating will give 5.6 kg of CaO? - Quora

Unical Science 2o22
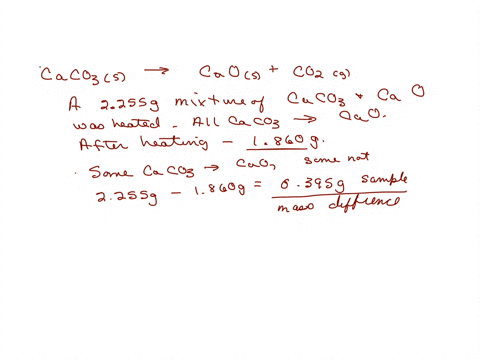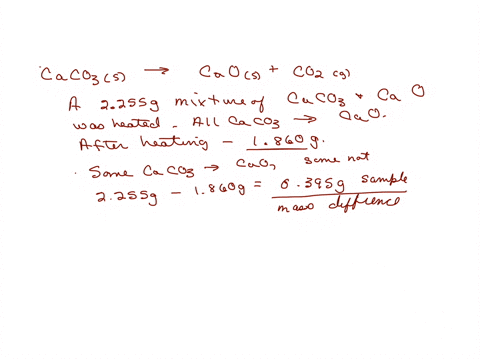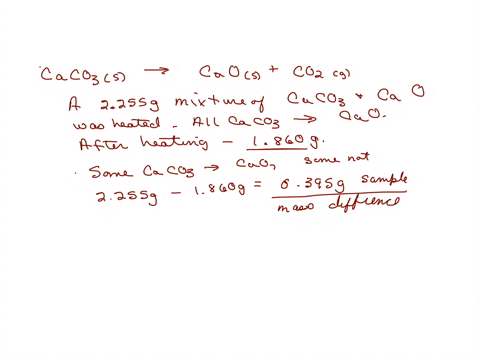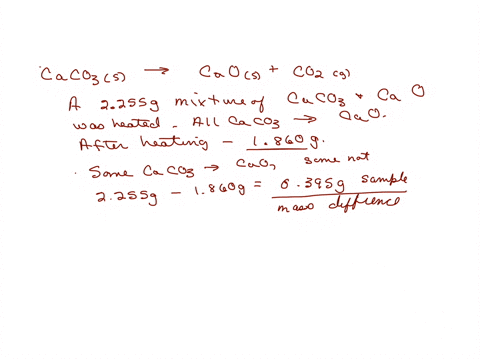
SOLVED: The weight percentage of limestone is as follows: CaCO3 = 93%, MgCO3 = 5%, Insoluble = 2%. This limestone is burned in a vertical kiln to produce lime (CaO, MgO, Insolubles).
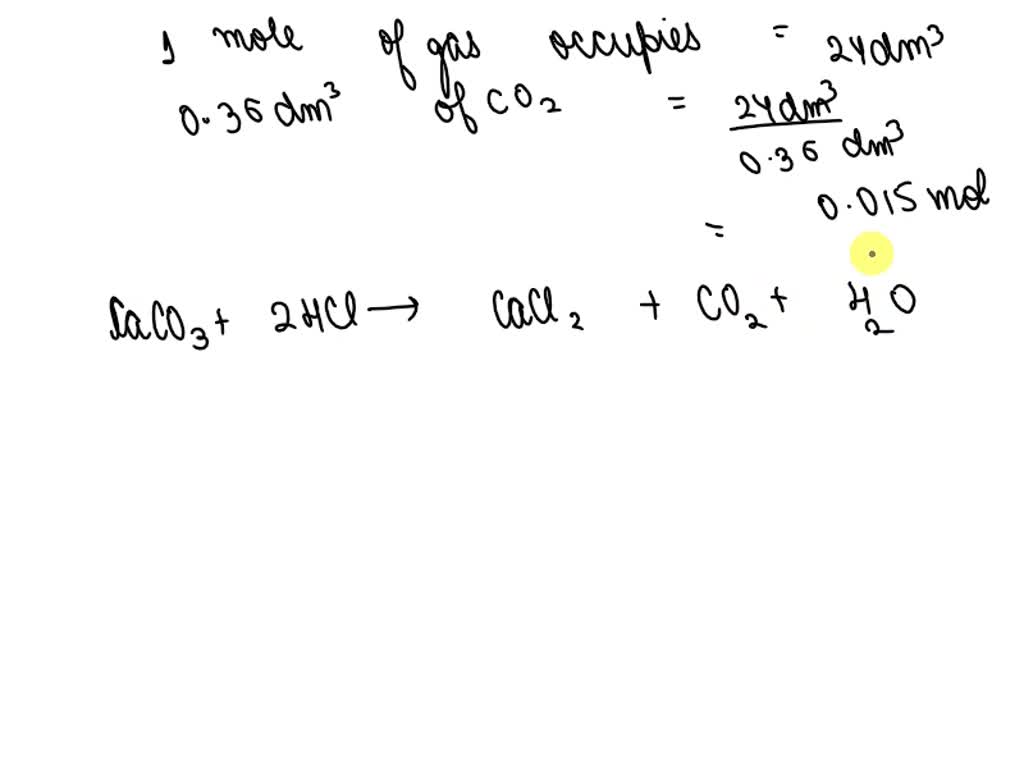
SOLVED: What is the percentage purity of the chalk? A sample of chalk ( limestone) with a mass of 1.70 g was reacted with excess HCl and the amount of carbon dioxide given

200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95

200g of a sample of limestone libetates 66g of CO2 on heating.The percentage impurity of CaCo3in the limestone is 1) 95

400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..
41. On thermal decomposition of 600g of lime stone 44.8L of co2 is released at STP. The percentage purity of lime stone is

Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources - Choi - 2009 - ChemSusChem - Wiley Online Library








