The vapour pressure of a solution having 2.0 g of solute X (gram
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
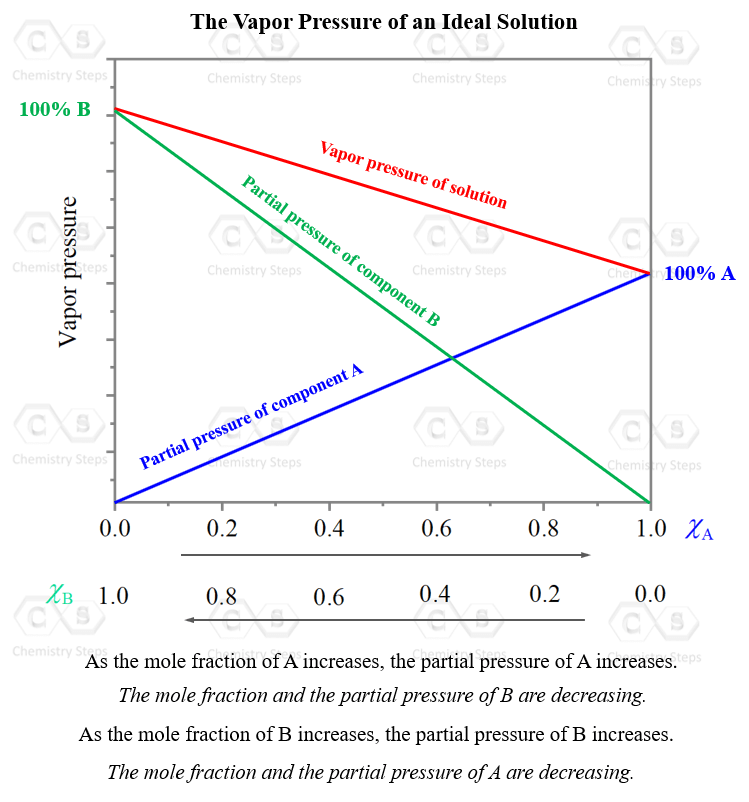
Vapor Pressure Lowering - Chemistry Steps

Composition profiles x 1 z and x 2 z as functions of the distance from

Solutions Homework Help, Questions with Solutions - Kunduz

The vapour pressure of a dilute aqueous solution of glucose is `700 mm` of `Hg` at `373 K`.

Coliyat 22. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 of CS, (vapour pressure = 854 torr) is 848.9
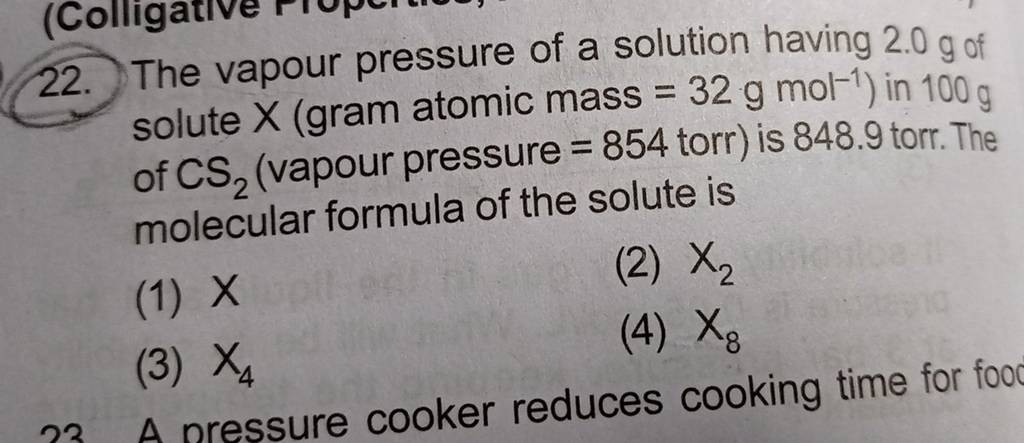
The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..

The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o

my lioperties, Abnormality in Molar Mass) 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g vapour pressure =

chapter 2

The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is 848.9 torr.








