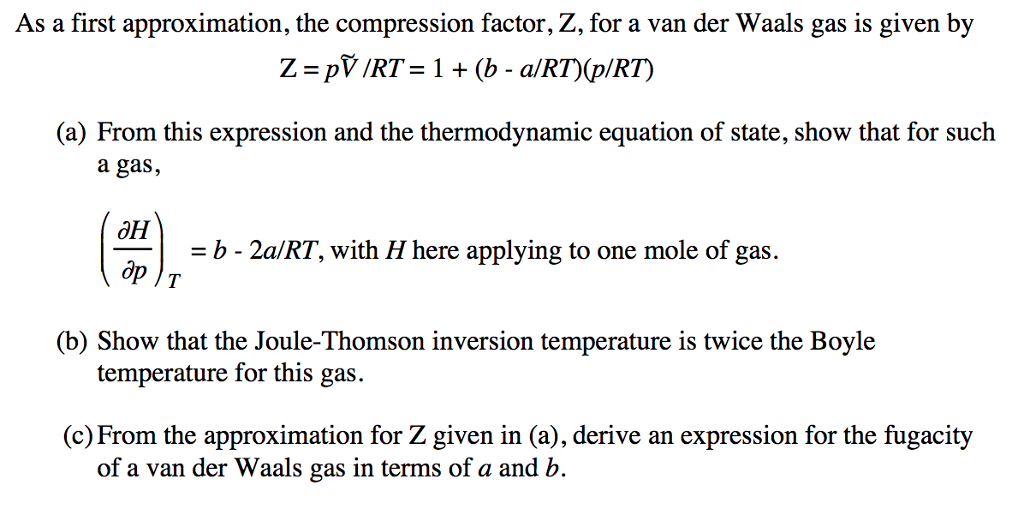
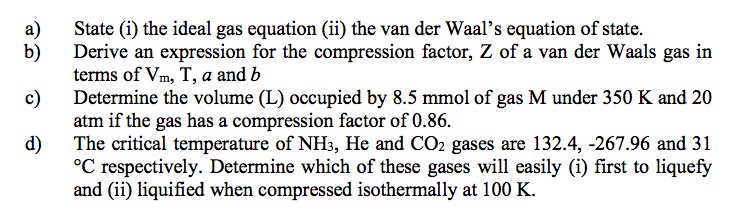
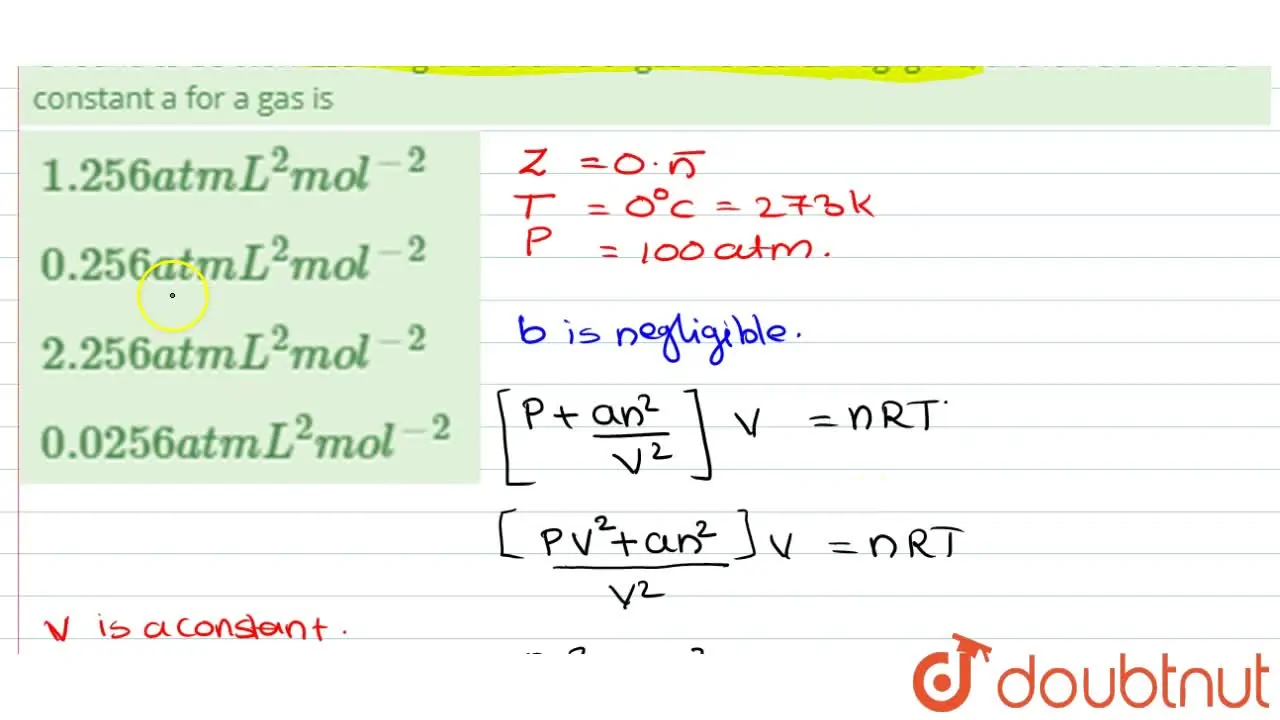
The compression factor (compressibility factor) for 1 mol of a van der
For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)
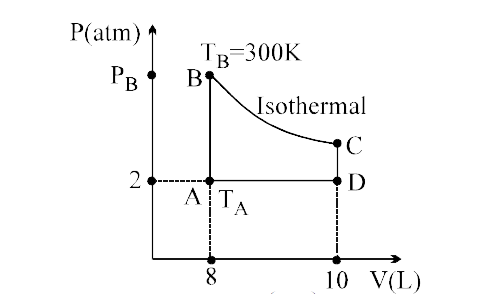
Temperature at A ((8)/( R )), Pressure at B ( 75R )
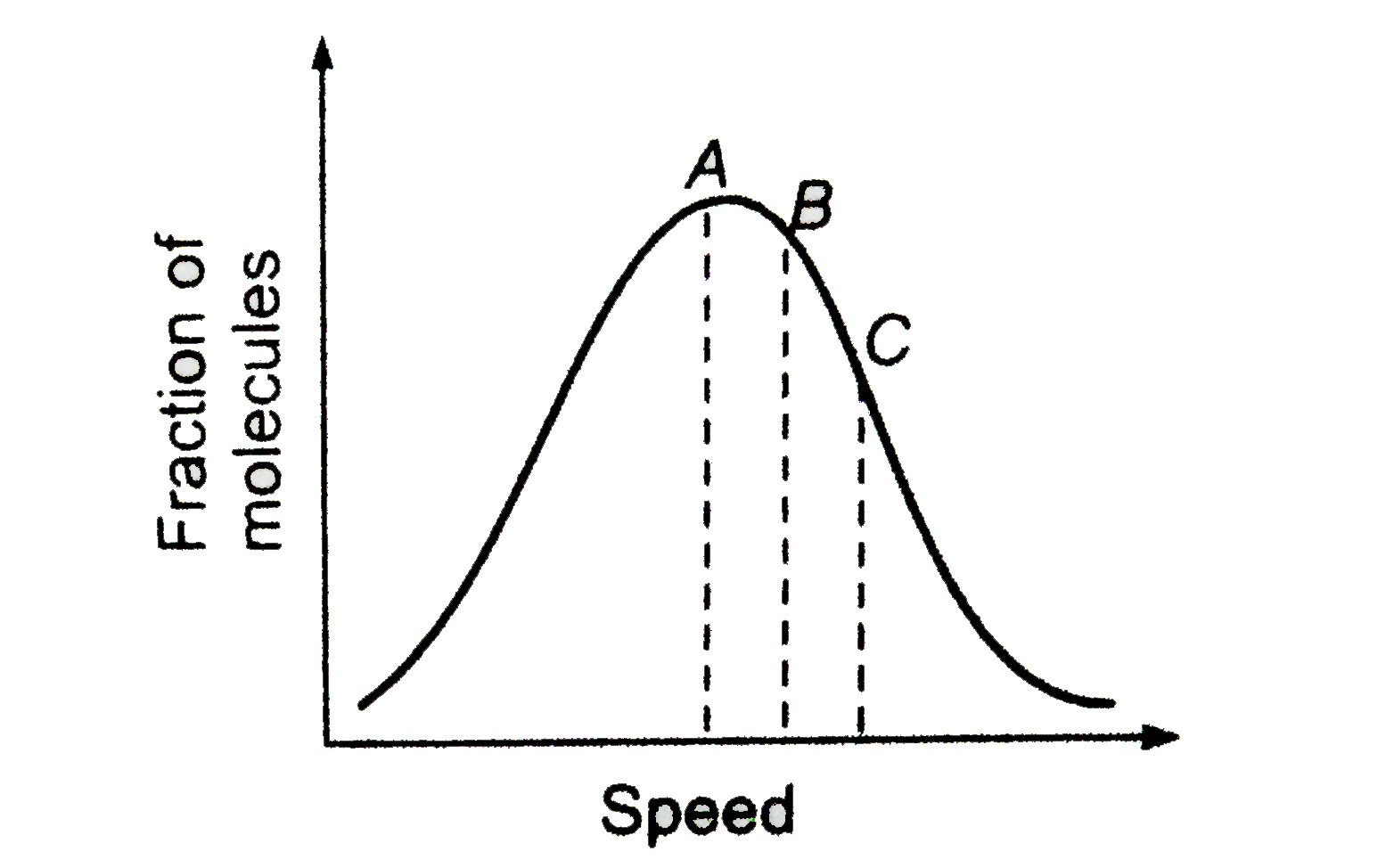
Distribution of molecules with velocity is represented by the curve

Two flask A and B have equal volume. Flask A contains H(2) and is main

If the volume occupied by CO(2) molecules is negligible, then calculat
The compressibility factor of gases is less than unity at STP. Therefo
Malayalam] The compressibility factor for definite amount of van der
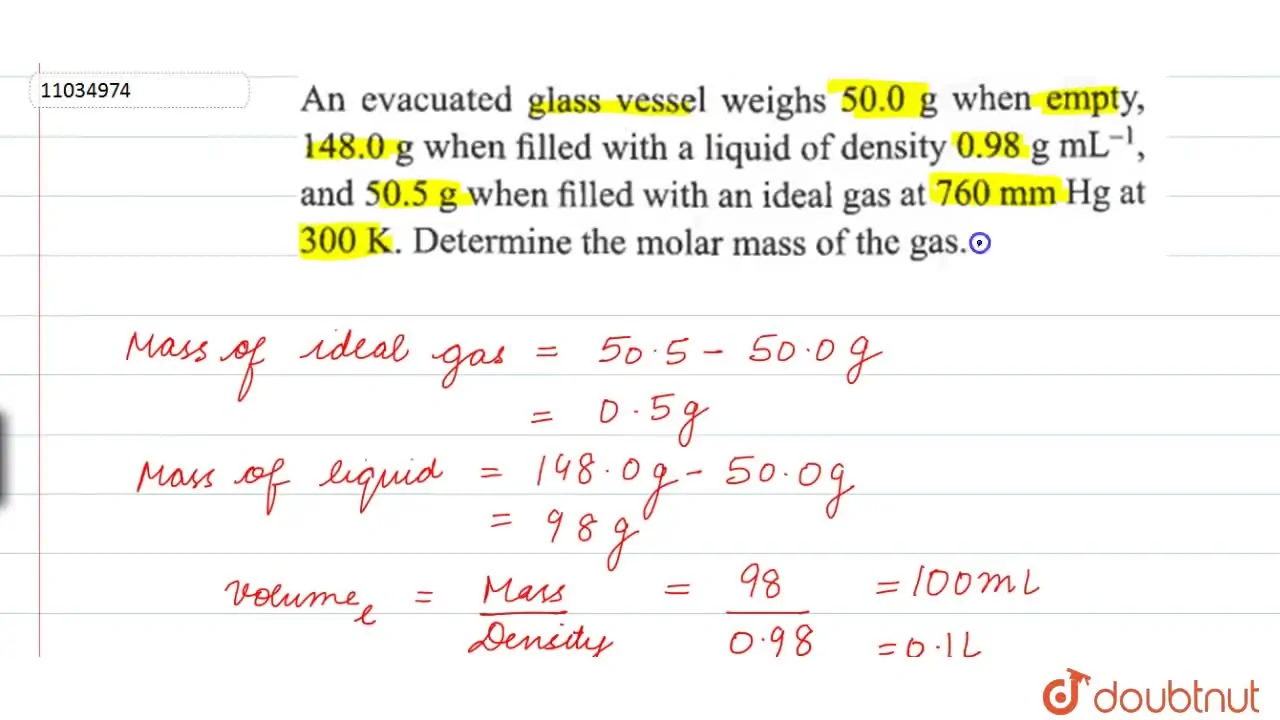
An evacuated glass vessel weighs 50.0 g when empty, 148.0 g when fille
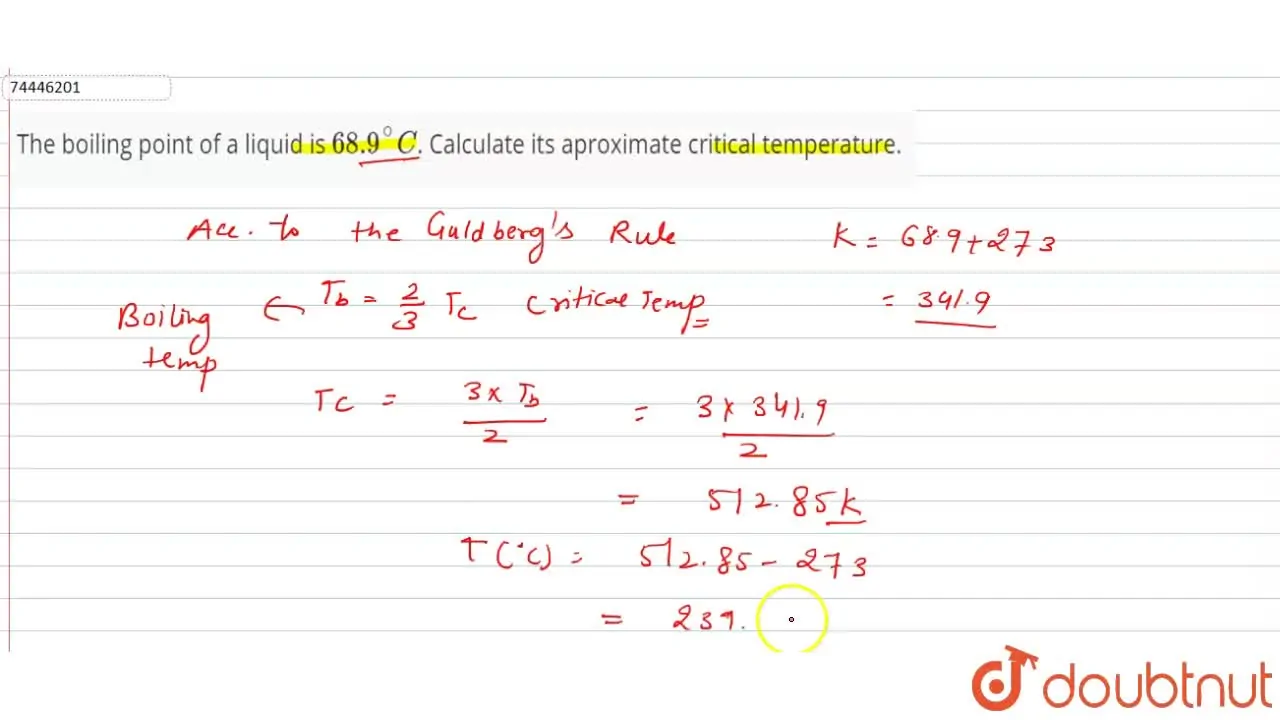
The boiling point of a liquid is 68.9^(@)C. Calculate its aproximate c
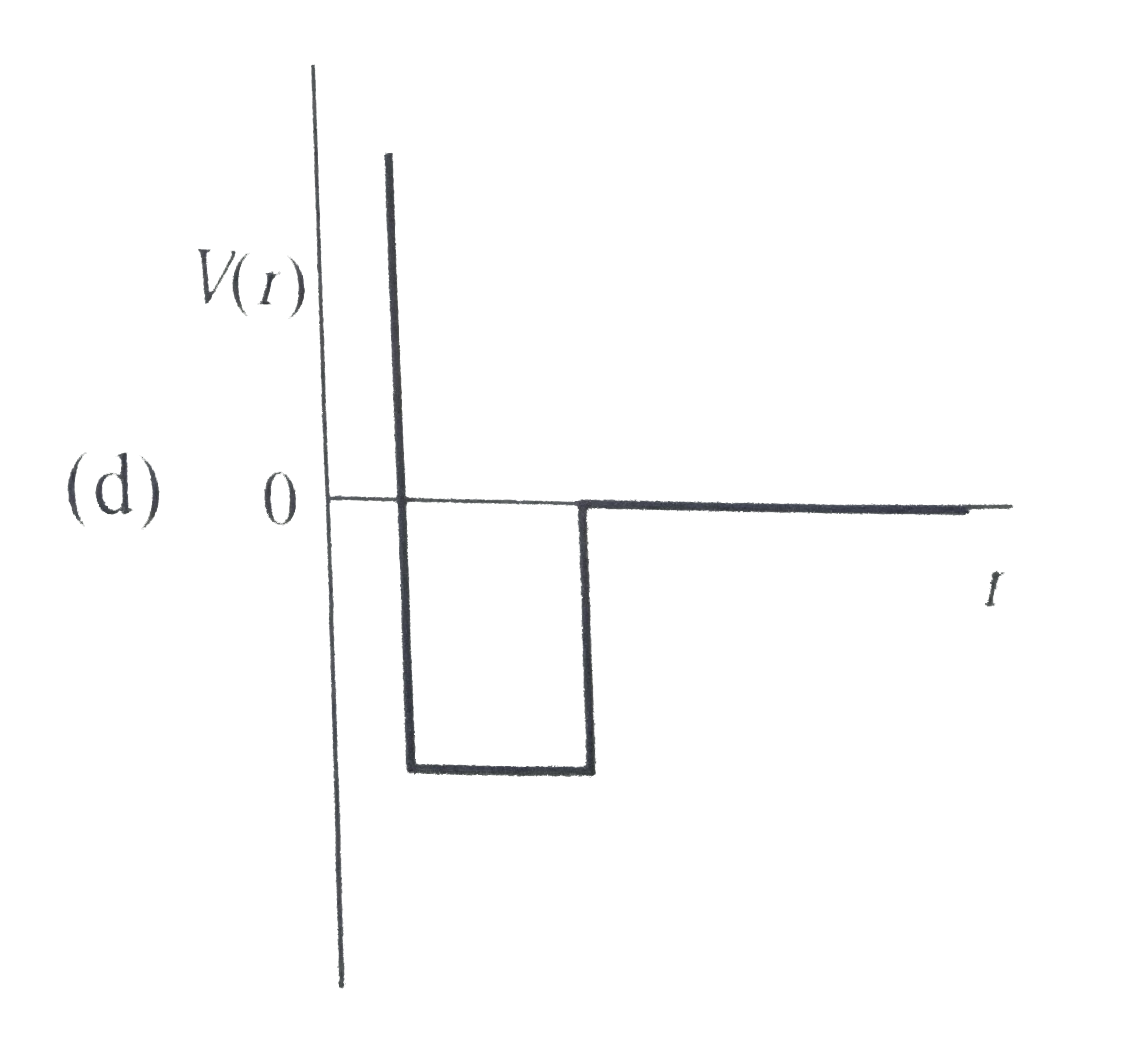
One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh

A mixture of ethane (C(2)H(6)) and ethene (C(2)H(4)) occupies 40 L at

Malayalam] The Compressibility factor for one mole of a van der Waal