Microbiological Media Management - SOP & Guideline - Pharma Beginners
Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.

SOP for Isolation and Identification of Microorganisms - Pharma Beginners
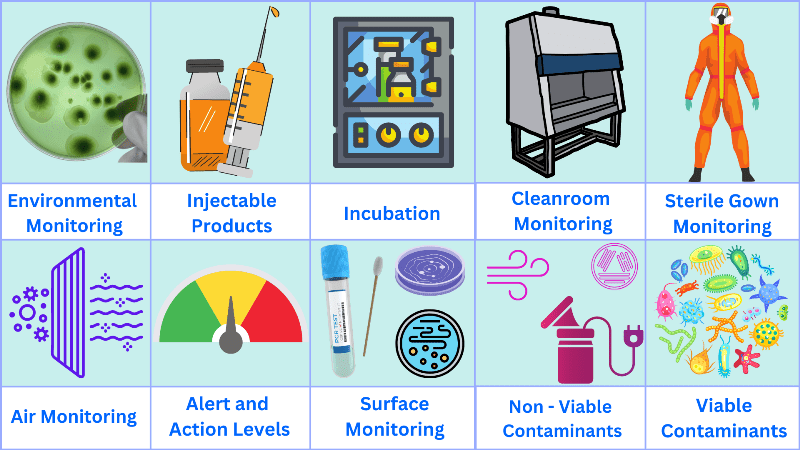
What is environmental monitoring in pharmaceutical industry

The Use of Microbiological Culture Media Article, PDF
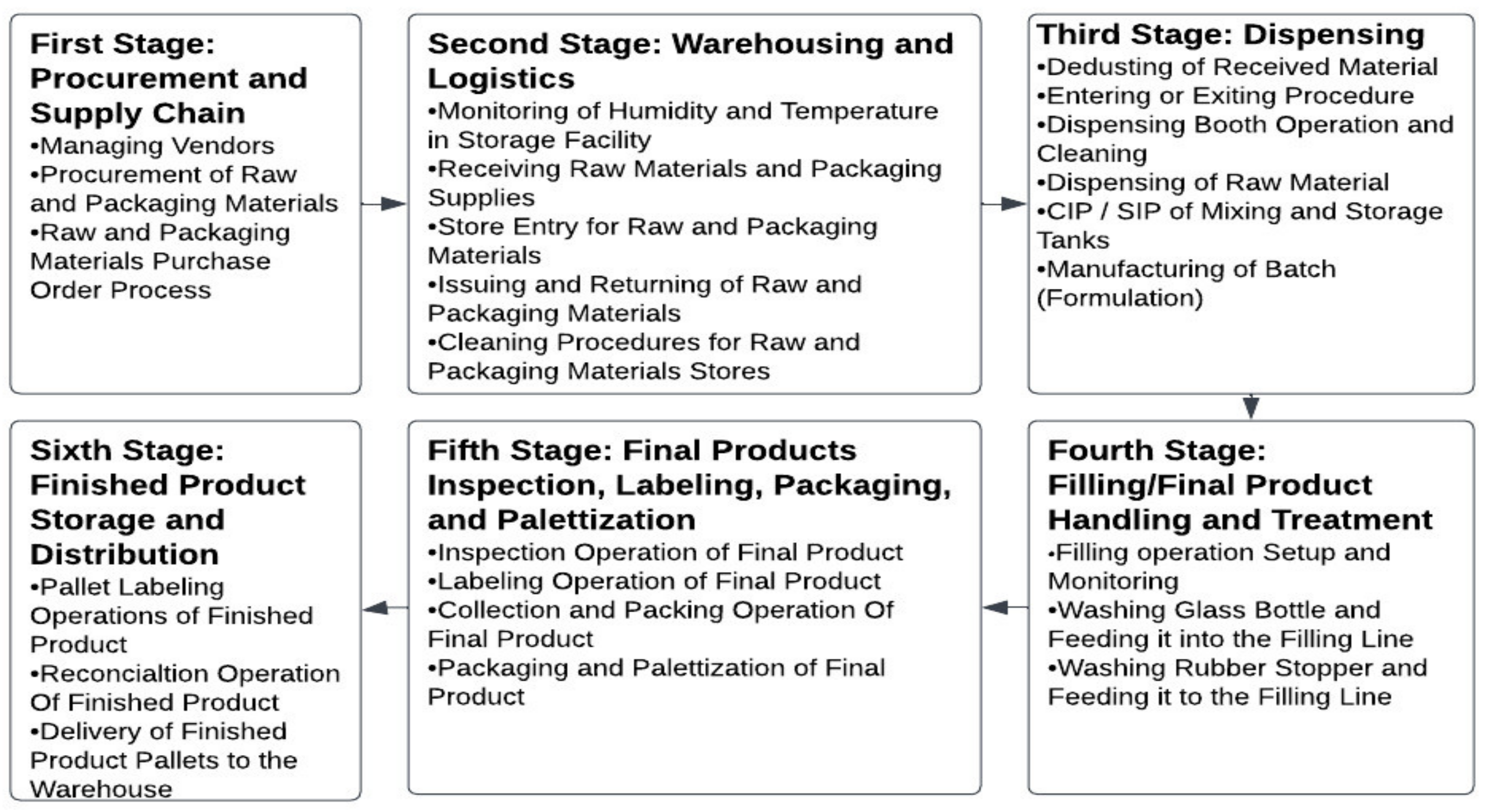
Sustainability, Free Full-Text
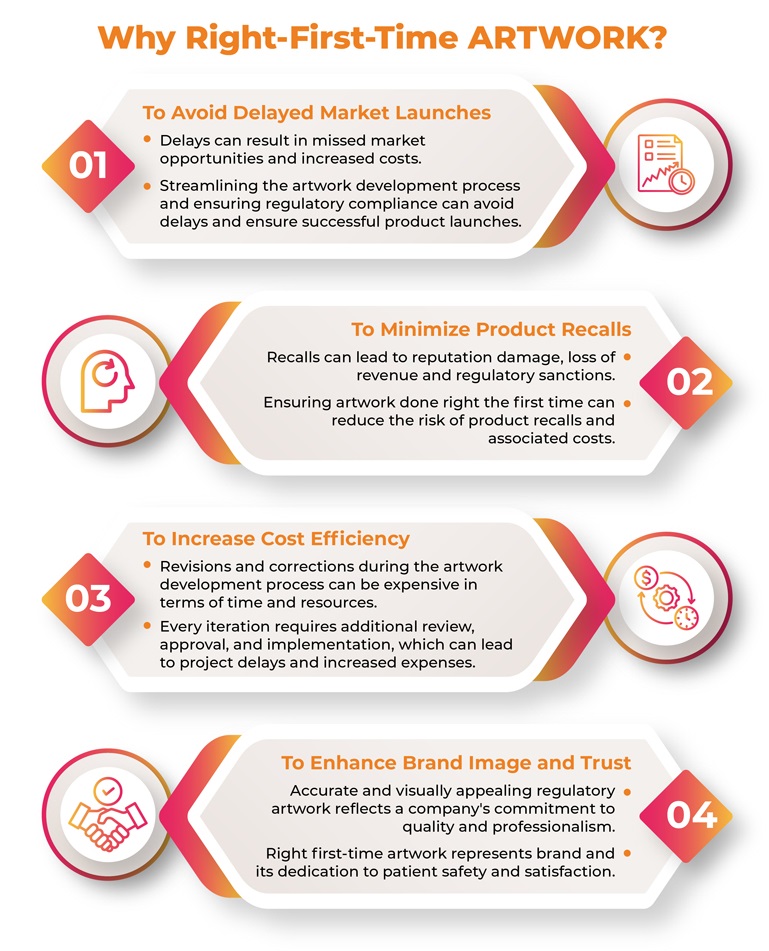
Guidance for Successful Pharmaceutical Artwork Management

Pharmaceutical Microbiology Resources: Microbiological Culture

The Importance of a Strong SOP System in the QC Microbiology Lab
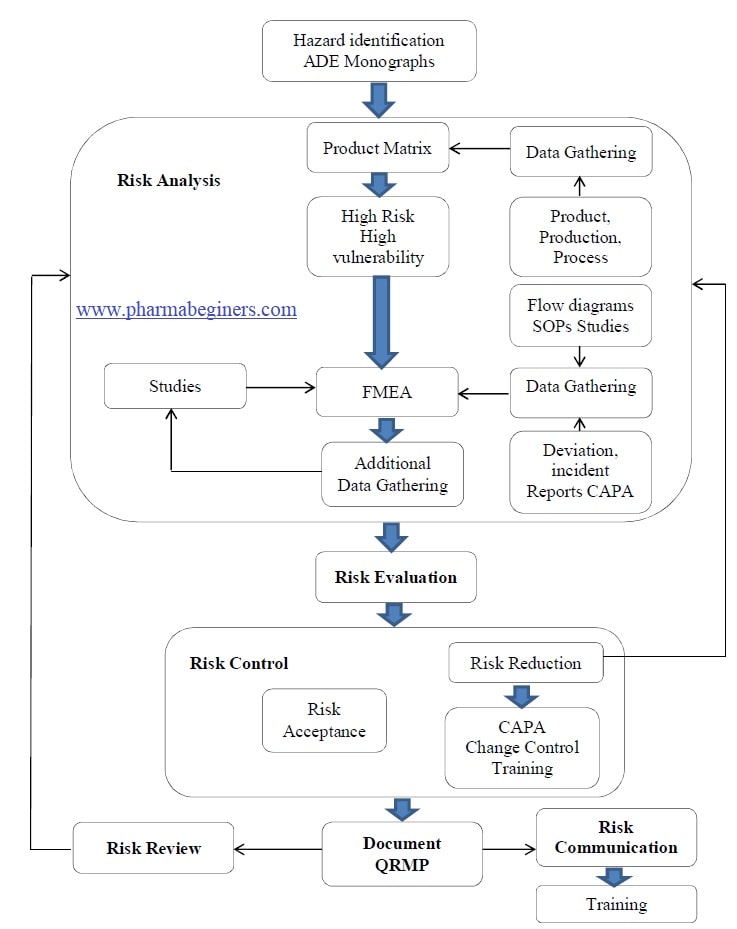
Cross Contamination, Mix-Ups & Microbial Contamination - SOP in Pharma

Culture Media Preparation

INTRODUCTORY OUTLOOK INTO ASEPTIC PROCESSING OF STERILE DOSAGE FORMS FOR PHARMACEUTICAL WORK
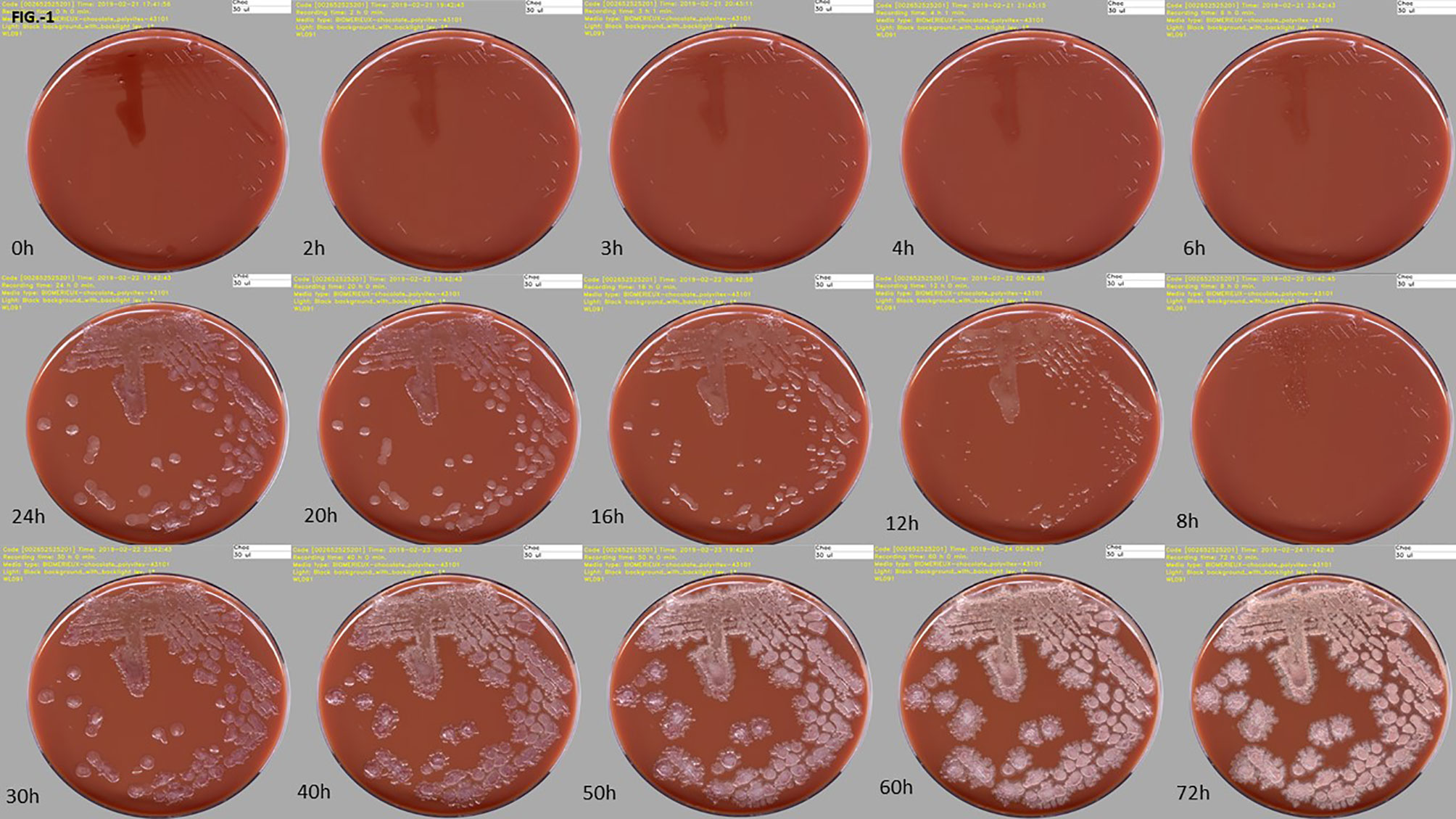
Frontiers Total Laboratory Automation for Rapid Detection and Identification of Microorganisms and Their Antimicrobial Resistance Profiles

Microbial Culture Media Preparation

Microbiological Culture Media: A Complete Guide for Pharmaceutical and Healthcare Manufacturers
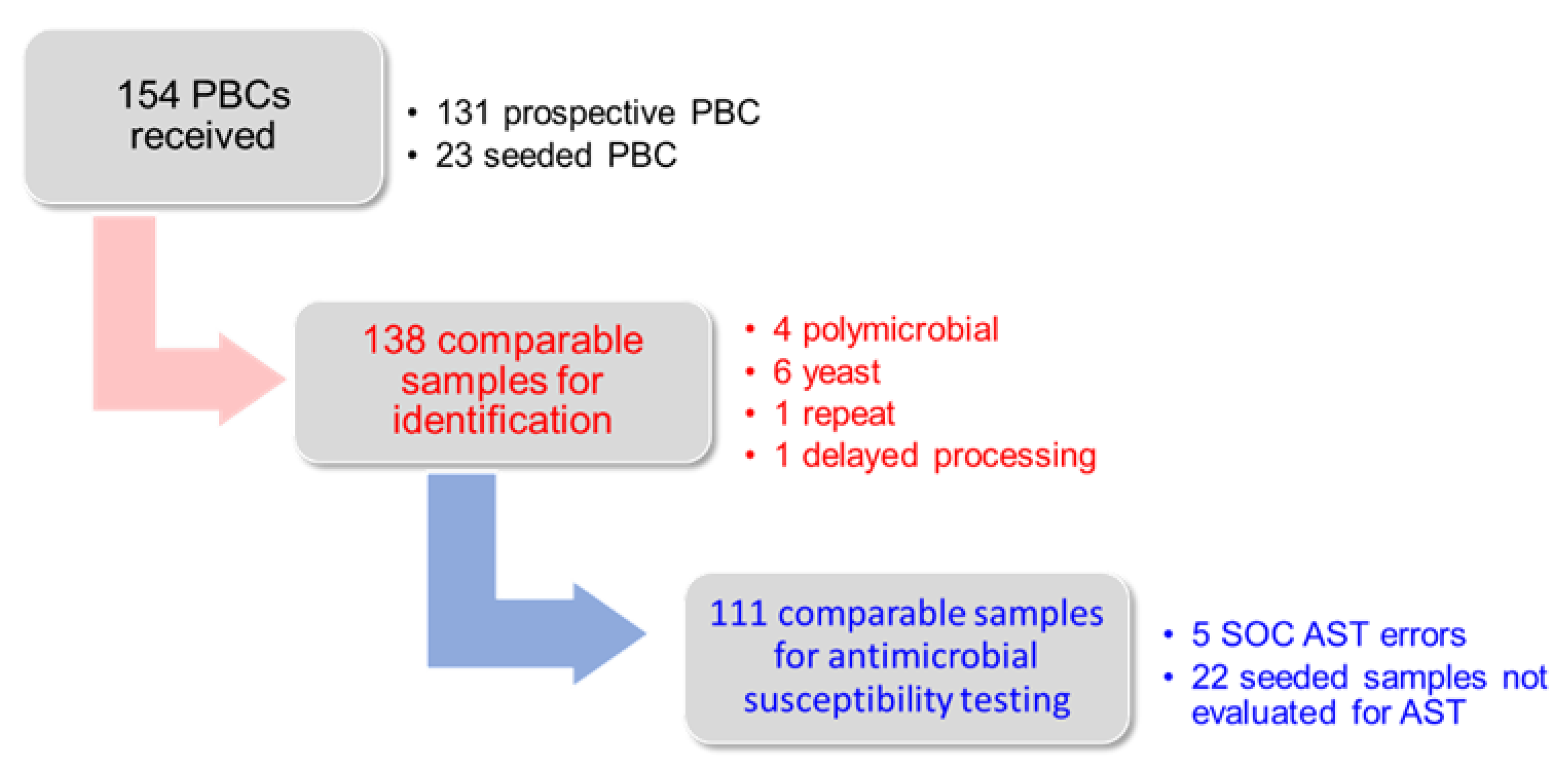
Microorganisms, Free Full-Text