Protocol for a randomised controlled feasibility trial of exercise
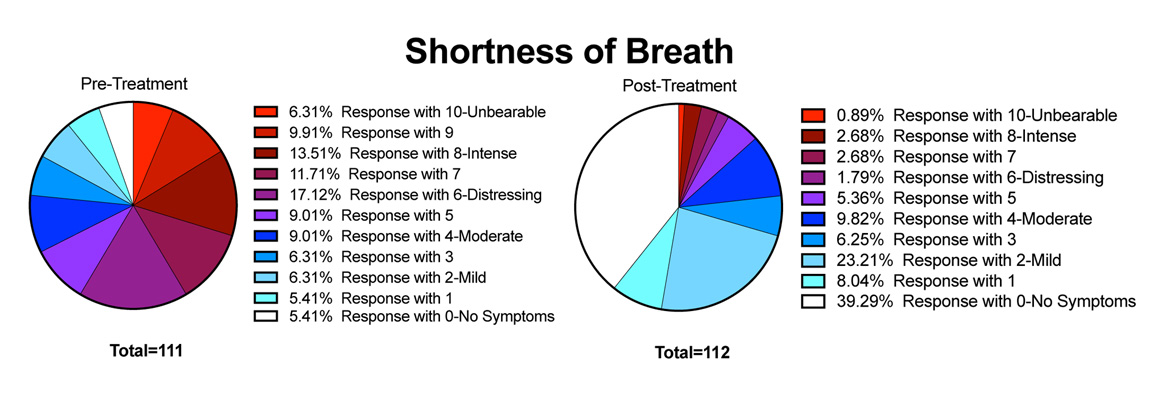
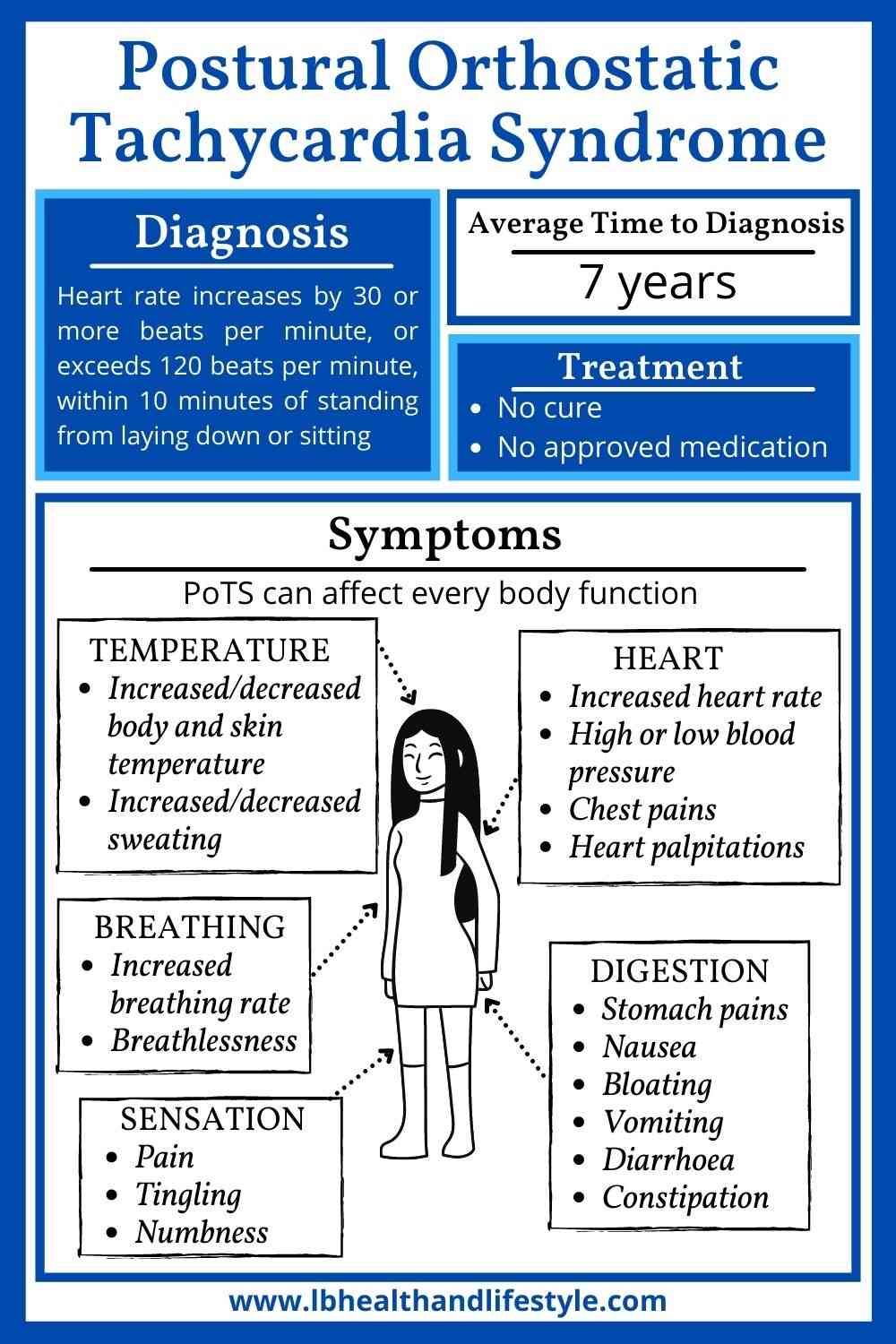
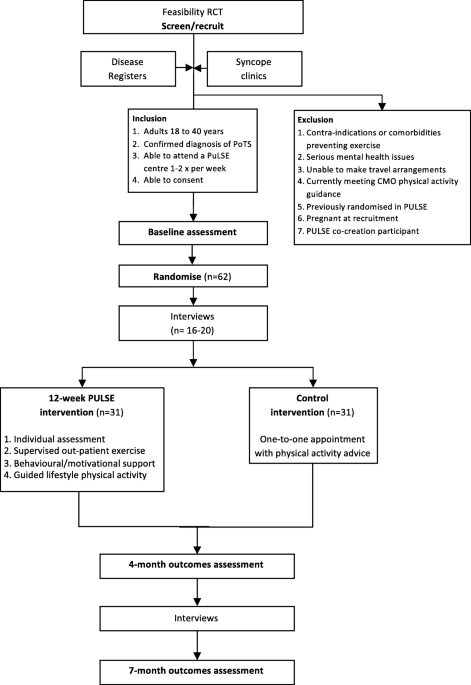
Background Postural orthostatic tachycardia syndrome (POTS) is an autonomic nervous system disorder causing an abnormal cardiovascular response to upright posture. It affects around 0.2% of the population, most commonly women aged 13 to 50 years. POTS can be debilitating; prolonged episodes of pre-syncope and fatigue can severely affect activities of daily living and health-related quality of life (HRQoL). Medical treatment is limited and not supported by randomised controlled trial (RCT) evidence. Lifestyle interventions are first-line treatment, including increased fluid and salt intake, compression tights and isometric counter-pressure manoeuvres to prevent fainting. Observational studies and small RCTs suggest exercise training may improve symptoms and HRQoL in POTS, but evidence quality is low. Methods Sixty-two people (aged 18–40 years) with a confirmed diagnosis of POTS will be invited to enrol on a feasibility RCT with embedded qualitative study. The primary outcome will be feasibility; process-related measures will include the number of people eligible, recruited, randomised and withdrawn, along with indicators of exercise programme adherence and acceptability. Secondary physiological, clinical and health-related outcomes including sub-maximal recumbent bike exercise test, active stand test and HRQoL will be measured at 4 and 7 months post-randomisation by researchers blinded to treatment allocation. The PostUraL tachycardia Syndrome Exercise (PULSE) intervention consists of (1) individual assessment; (2) 12-week, once to twice-weekly, supervised out-patient exercise training; (3) behavioural and motivational support; and (4) guided lifestyle physical activity. The control intervention will be best-practice usual care with a single 30-min, one-to-one practitioner appointment, and general advice on safe and effective physical activity. For the embedded qualitative study, participants (n = 10 intervention, n = 10 control) will be interviewed at baseline and 4 months post-randomisation to assess acceptability and the feasibility of progressing to a definitive trial. Discussion There is very little high-quality research investigating exercise rehabilitation for people with POTS. The PULSE study will be the first randomised trial to assess the feasibility of conducting a definitive multicentre RCT testing supervised exercise rehabilitation with behavioural and motivational support, compared to best-practice usual care, for people with POTS. Trial registration ISRCTN45323485 registered on 7 April 2020.

PDF) Tailored exercise management (TEMPO) versus usual care for people aged 80 years or older with hip/knee osteoarthritis: study protocol for a feasibility randomised controlled trial

Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases - Pedersen - 2015 - Scandinavian Journal of Medicine & Science in Sports - Wiley Online Library

Schedule of enrolment, interventions and assessments

World Health Organization trial registration data set

PDF) Randomised Controlled Feasibility Trial of an Evidence-Informed Behavioural Intervention for Obese Adults with Additional Risk Factors
Feasibility of a high-PRotein Mediterranean-style diet and resistance Exercise in cardiac Rehabilitation patients with sarcopenic obesity (PRiMER): Study protocol for a randomised control trial

Congratulations: Dr Mary Abboah-Offei - Cicely Saunders International

Annals of Surgical Oncology - Society of Surgical Oncology

Feasibility & Study Start-Up at SCOPE Summit