
Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel
Groundbreaking technology introduces increased control to achieve optimal coverage proven to significantly reduce the risk of toxicity to the rectum SANTA BARBARA, CALIF. / STOCKHOLM, SWEDEN – June 9, 2022— Palette Life Sciences, a fully-integrated global life sciences company dedicated to improving patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of […]

Barrigel on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…
FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences

Spectrum Laboratory Products, Inc. Issues Voluntary Worldwide Recall of Epinephrine (L-Adrenaline) USP Bulk Active Pharmaceutical Ingredient (API) Due to Discoloration of Product

Palette Life Sciences Enters Into Definitive Agreement with Teleflex Incorporated
Next Generation Blood Collection and Separation Technology Receives FDA 510( k) Clearance

David Aguilar on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

David Aguilar on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

Marianna Hawkins posted on LinkedIn

Vinny Devany on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…
SpaceOAR - Augmenix, Boston Scientific, and Conflicts of Interest, Page 4

Helena Jansson på LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…
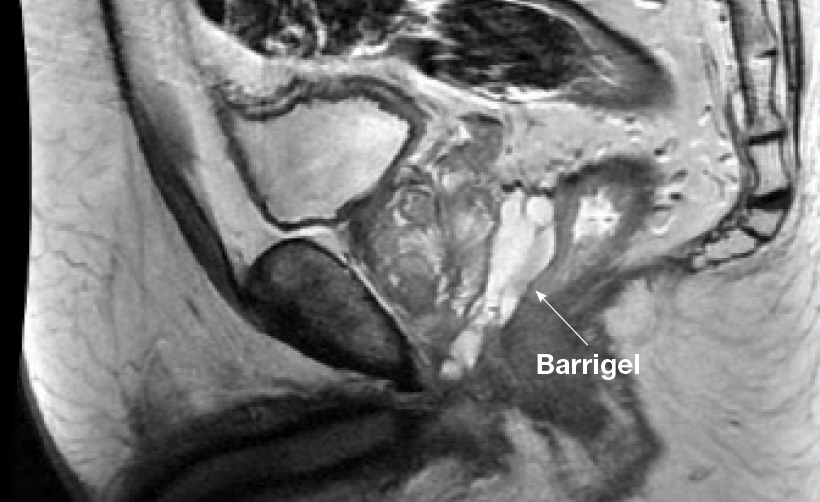
Control Matters Barrigel for Healthcare Providers
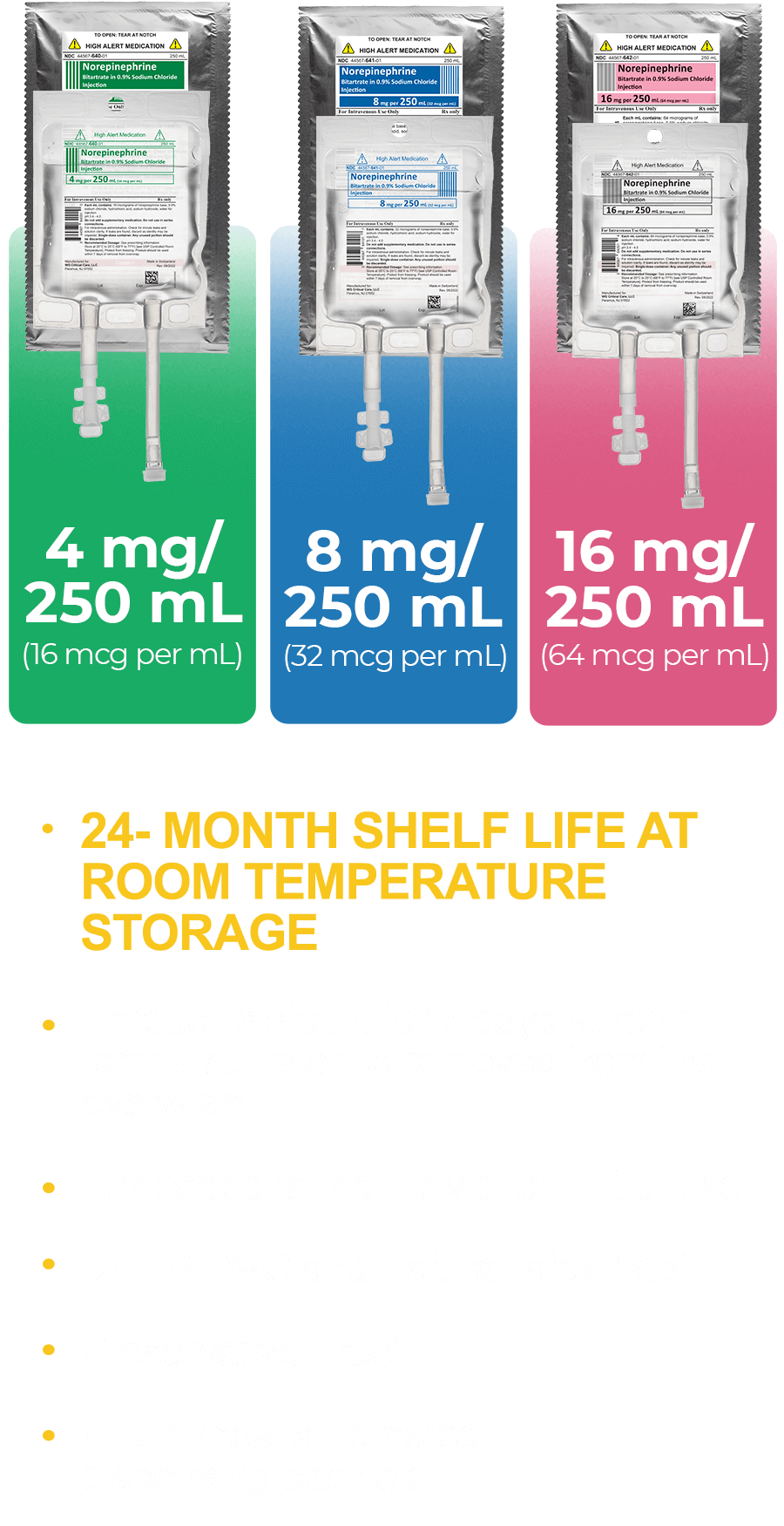
Norepinephrine Bitartrate in 0.9% Sodium Chloride Injection – WG Critical Care
益生菌活化出新技术,肠道存活率提高1000倍?

Pratik Patel on LinkedIn: #barrigel #prostatecancer #firstinarizona #gamechanger









