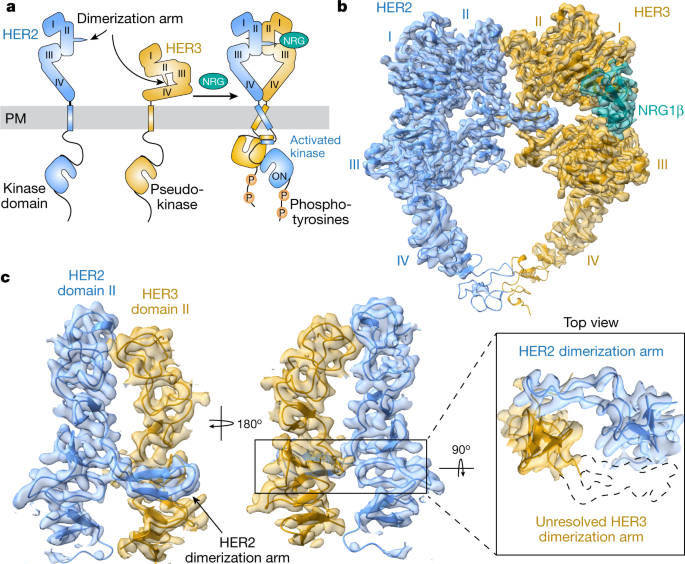
Structures of the HER2–HER3–NRG1β complex reveal a dynamic dimer

SEC profiles showing the formation of HER2-trastuzumab-pertuzumab

Natalia JURA, UCSF University of California, San Francisco, CA, UCSF, Cardiovascular Research Institute

A molecular mechanism for the generation of ligand-dependent differential outputs by the epidermal growth factor receptor. - Abstract - Europe PMC
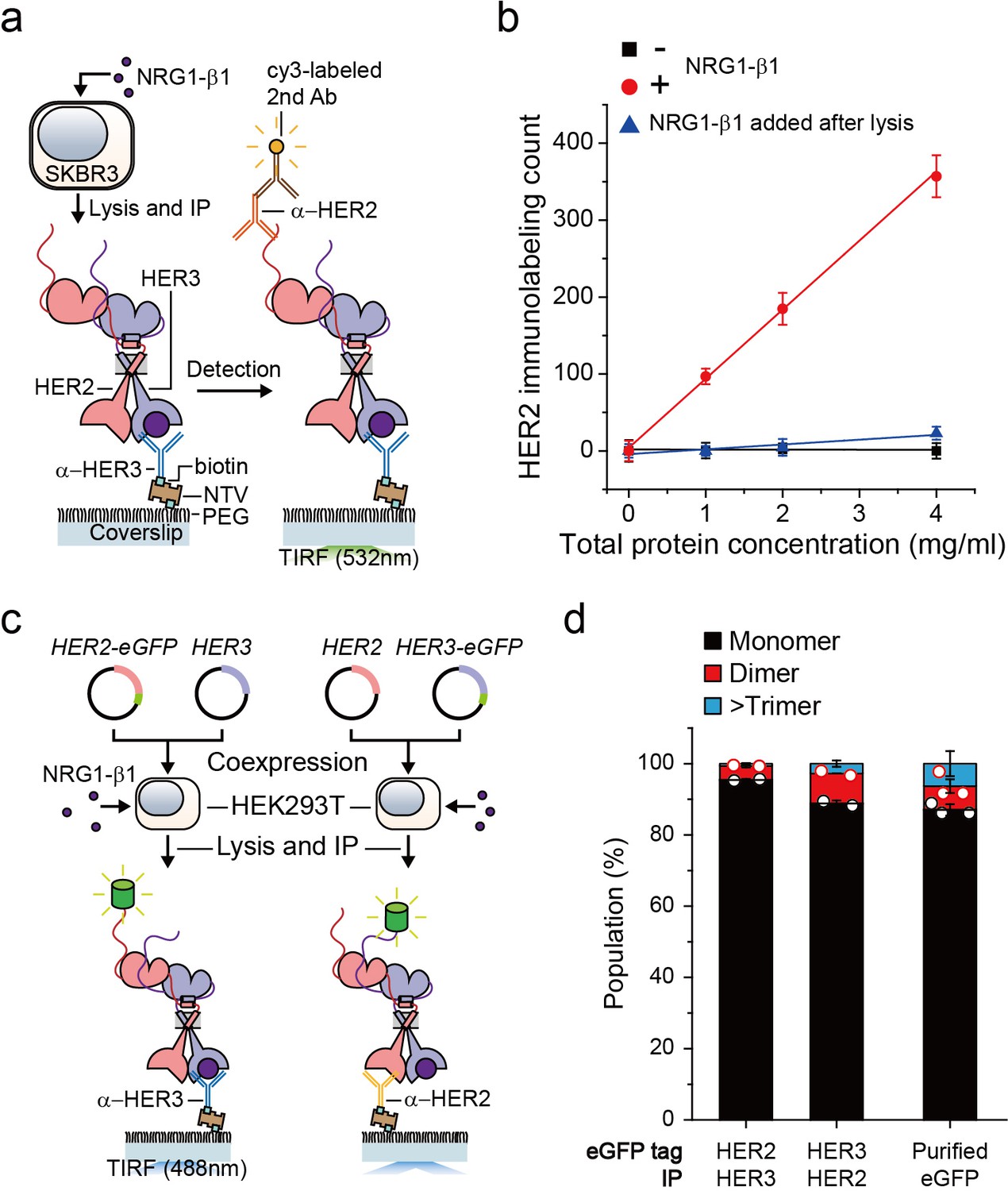
Single-molecule functional anatomy of endogenous HER2-HER3

Full article: Enhanced Binding at Fever Temperatures of HER2 in Complex with Trastuzumab and Pertuzumab

In-depth structural and functional analysis of the NRG1β binding

The tEGFR:TGFα domain II dimer interface is similar to the

Mechanisms of Receptor Tyrosine-Protein Kinase ErbB-3 (ERBB3

Association of patient/tumor characteristics with HER2 somatic mutation

Molecular dynamics simulations of asymmetric heterodimers of HER1

Structural dynamics of the active HER4 and HER2/HER4 complexes is

The fixed extended conformation of ErbB2 precludes formation of