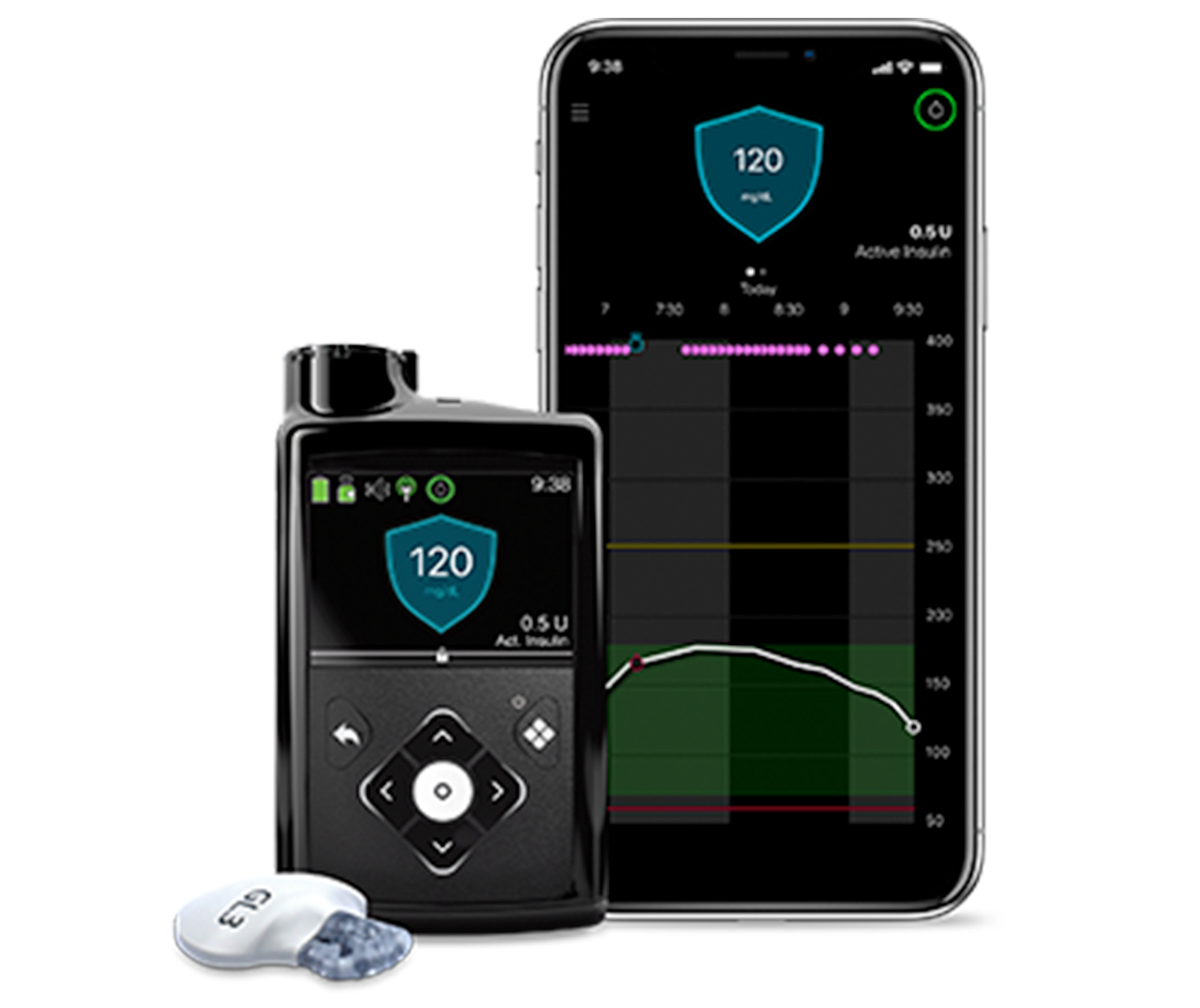
Modular Medical submits next-gen insulin pump for FDA clearance
4.6
(459)
Write Review
More
$ 14.99
In stock
Description
Modular Medical (Nasdaq:MODD) announced today that it submitted its next-generation MODD1 insulin pump to the FDA for 510(k) clearance.

as-ex99_1page004.jpg

Diabetes Business The Savvy Diabetic

Vascular Graft Solutions wins FDA approval for clampless proximal anastomosis - MassDevice

Cook, AcuityBio ink deal for lung cancer drug technology - MassDevice
Planet MicroCap

New Diabetes Technology Coming in 2022

FDA clears icometrix' MRI quantification software - MassDevice

Novocure touts combined TTF, chemotherapy mesothelioma study data - MassDevice

Drug Delivery Business News on LinkedIn: Medicare covers Medtronic MiniMed 780G automated insulin pump
You may also like