:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
How to Calculate Normality of a Solution
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.
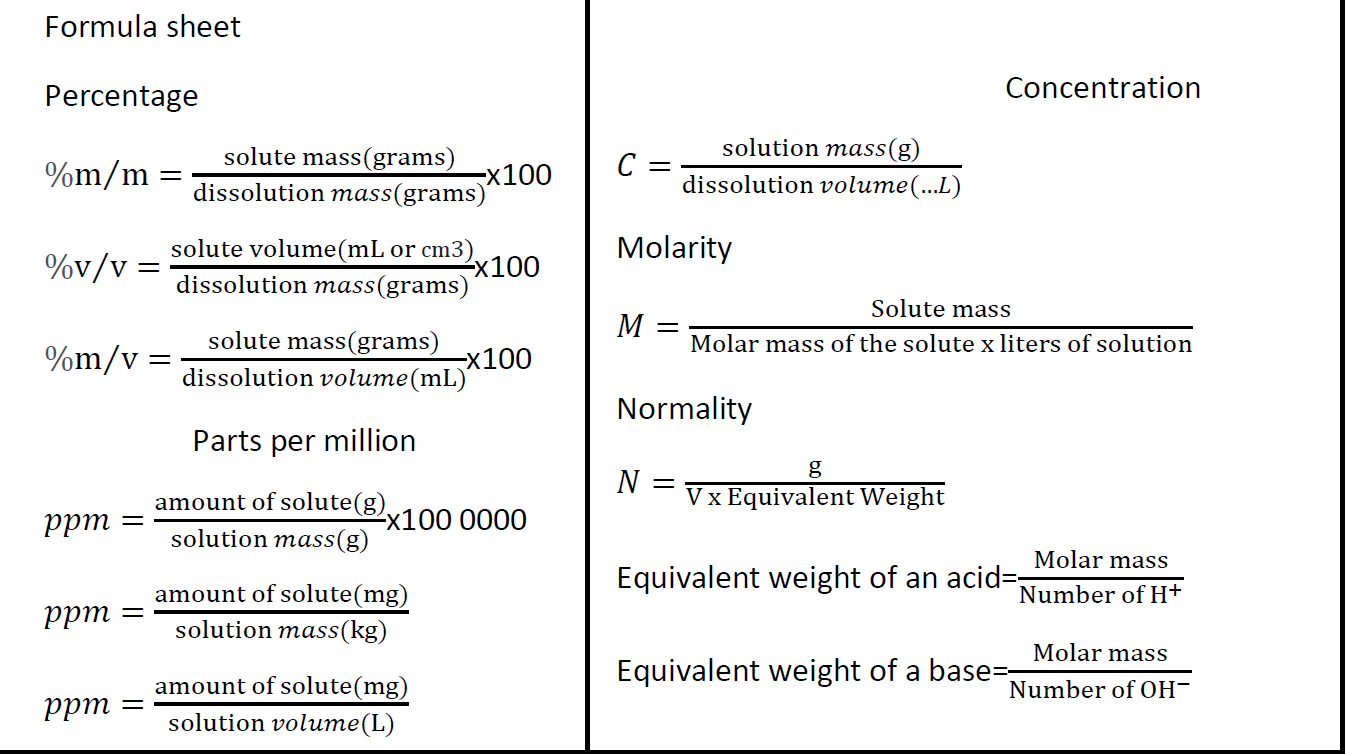
Molarity and Normality (CHEMISTRY) They are simple

Solved Examples Ex-8. Calculate the normality of a solution containing 15.8 g of KMnO4 in 50 mL acidic solution. W x 1000
:max_bytes(150000):strip_icc()/scientist-performing-experiment-530887598-580f6db25f9b58564ccb5bcb.jpg)
How to Calculate Normality of a Solution

11) 1000 (2) TUU 21. Normality of 10% (wV) H,SO, solution is nearly (1) 0.1 (2) 0.2 (3) 0.5 (4) 2

How can the normality of concentrated sulphuric acid be determined? - Quora
What is the molarity of 0.4 normality of H2SO4 solution? - Quora

normalitymeaninginchemistry

Normality-Measuring the Concentration of an Element
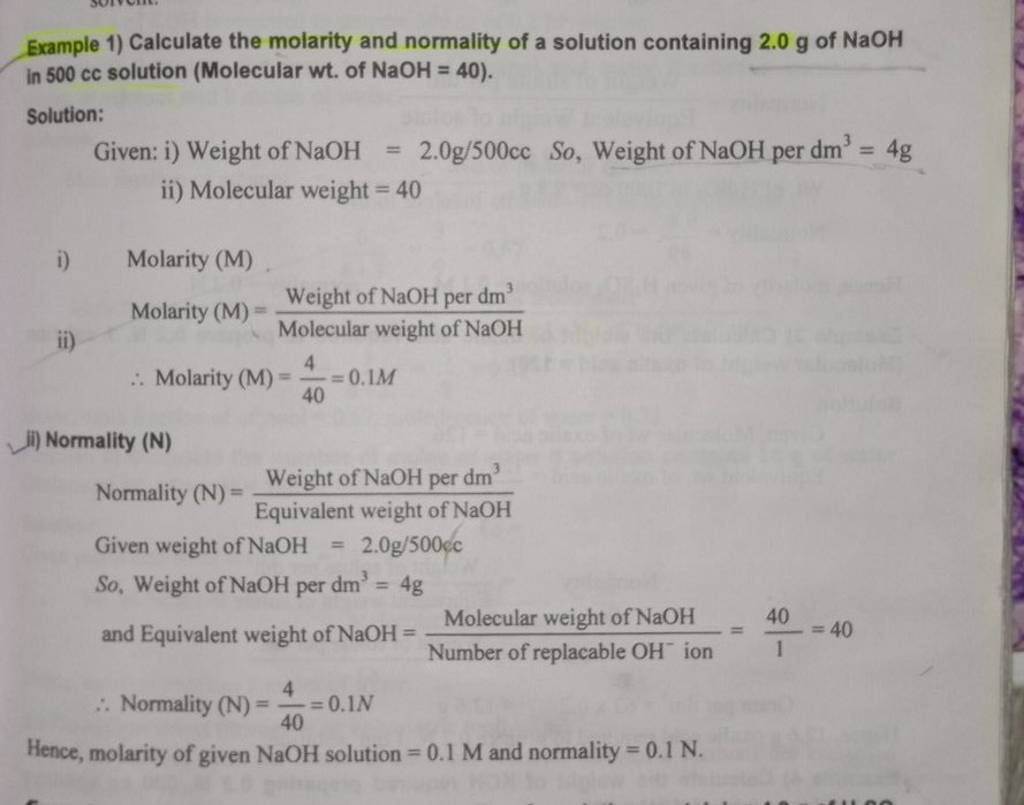
Example 1) Calculate the molarity and normality of a solution containing ..

SOLUTION: Difference between molarity molality and normality and how to calculate them - Studypool

16) Calculate normality of a solution in which 1.2×1024OH−ions are prese..
:max_bytes(150000):strip_icc()/Normality-58c08dbd5f9b58af5c93b56a.jpg)
How to Calculate Normality of a Solution
Untitled Document

My Smart Class : How To Calculate Normality
Calculate the normality of a solution containing 62.3 g of hydrated copper sulphate in 500ml of solution (Cu= 63, S= 32, O=16, H=1)