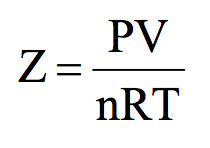117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum
Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum

PCAST Vol 2, Ch 3 Engineering - The FIRE Place

Industry Europe – Issue 27.4 by IndustryEurope - Issuu

Compressibility factor for H2 behaving as real gas is

Compressibility factor (gases) - Citizendium

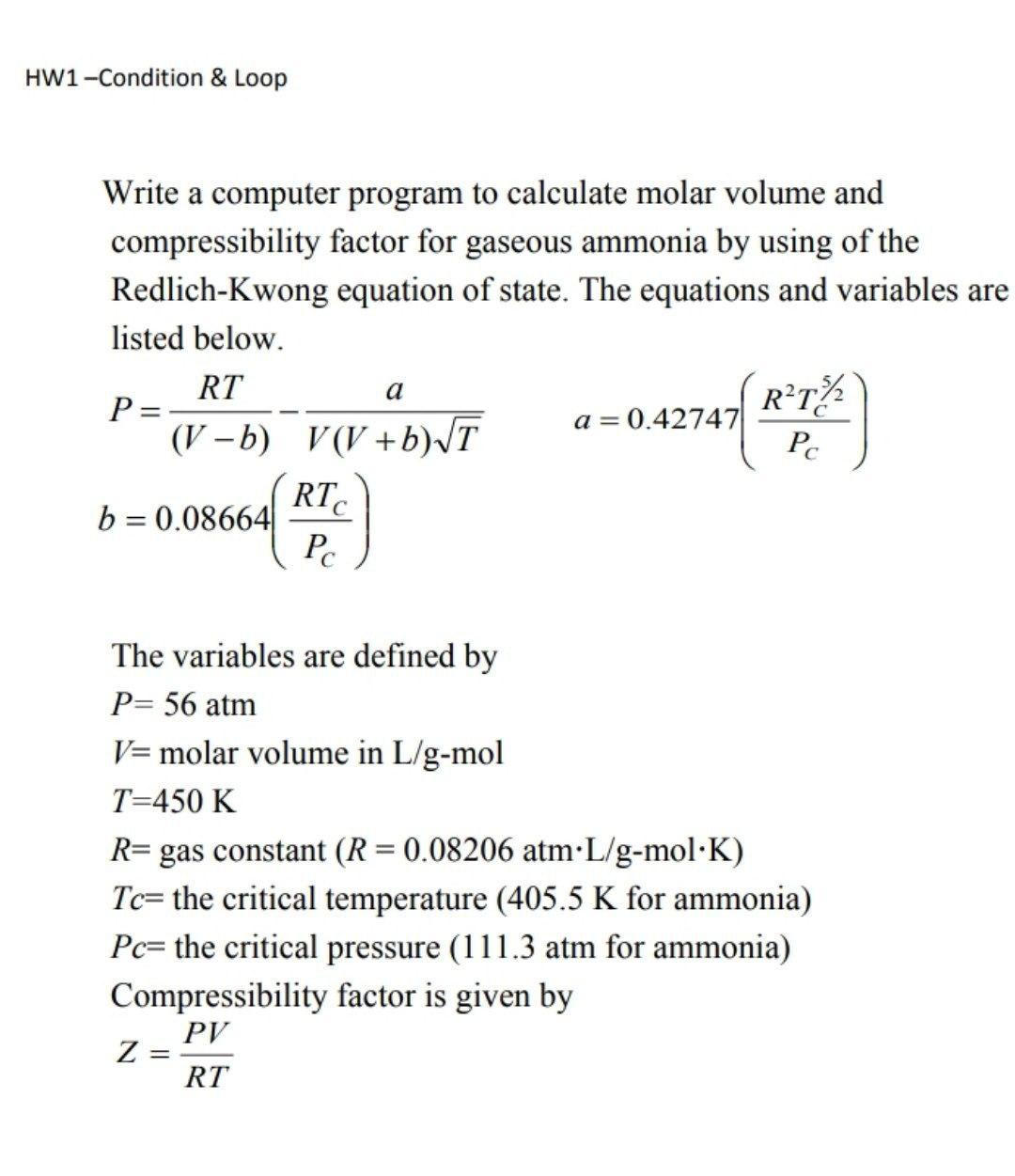
ME2036- ENGINEERING THERMODYNAMICS BY Mr.P.SATHISH

159. A gas 350 K and 15 bar has molar volume 20 percent smaller than that an ideal gas under the same conditions. The correct option about the gas and its compressibility

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor is given as (1) - RTV Pb RT 12 12 Photo (3) 1+ TV Pb RT 3. 10 mole of an ideal gas 27°C ernands

Solved The plot below shows how compressibility factor (Z)

Real Gas Behavior The Compression Factor (Z) [Example #2]

Solved The graph of compressibility factor (Z)v/sP for 1 mol
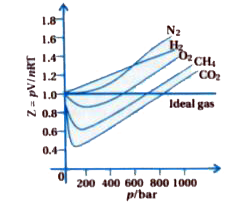
Gujrati] Explain compressibility factor (Z).
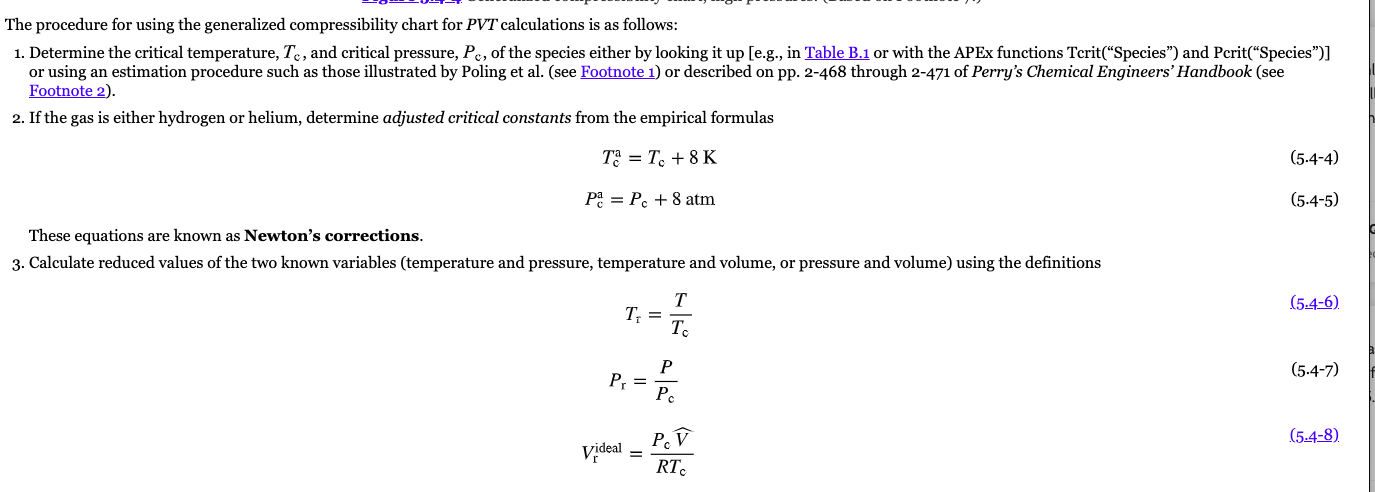
Solved Use the compressibility charts to answer the

Compressibility factor for H_2 behaving as real gas is : (1) 1 (2) (1-a/RTV) (3) (1+Pb/RT) (4) RT

For one mole of a real gas, curves are plotted under different conditions the same temperature as shown in diagram: slope = 102 2463 C (In low pressure region) RT slope =