Ideal and Real Gases - Definition, Comparison, Properties
Ideal and real gases definition, properties, comparison by ideal gas equation, thermal expansion, compressibility factor formula graph Boyle temperature
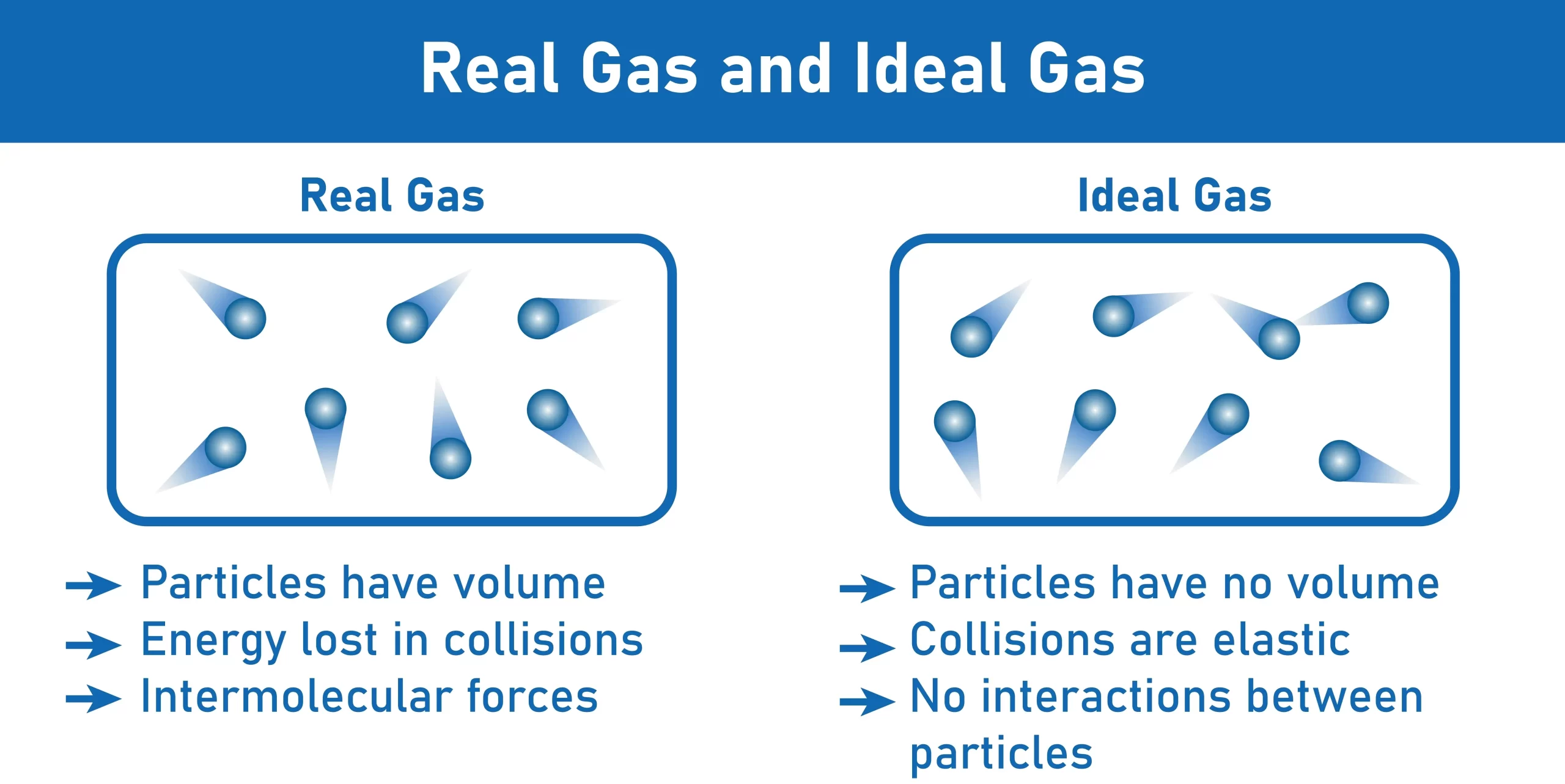
Difference between Ideal Gas And Real Gas

Differentiate between ideal gases and real gases.

Properties of matter: Gases

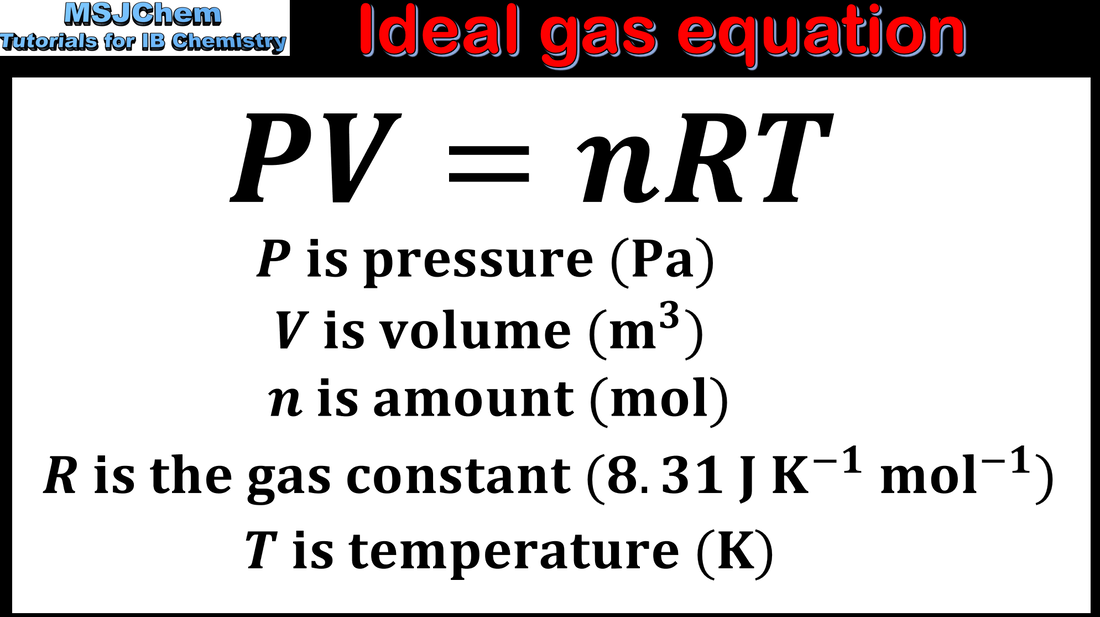
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas

Chem B M. Shell Spring Review What is the ideal gas law? PV = nRT
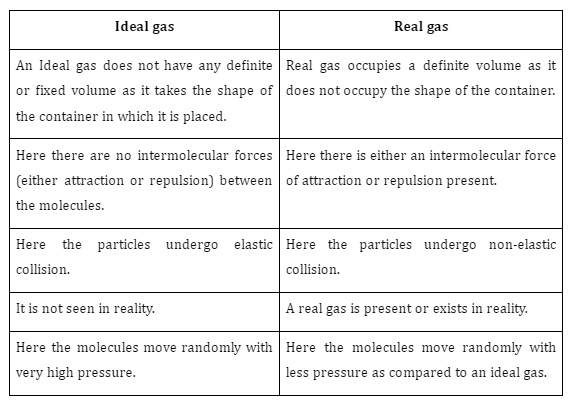
Difference between Ideal Gas And Real Gas

Chapter 10 Gas Laws Objectives: Understand the characteristics of

Pressure, Temperature & Volume of a Gas

Molar Mass & Ideal Gas Law Overview, Formula & Examples - Lesson

Kinetic Theory Of Gases - Explanation, Assumptions, Postulates

Chemical Elements & Compounds

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas

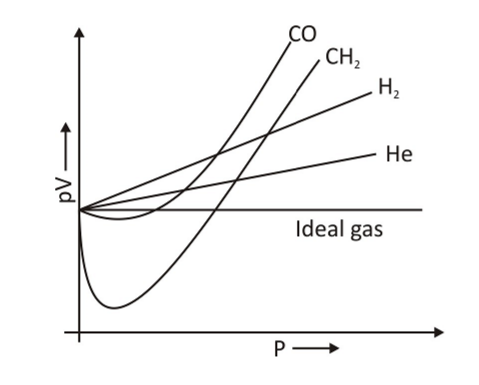
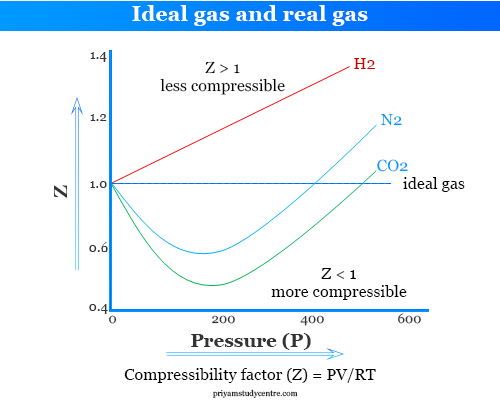
Deviations from Ideal Gas Law Behavior

Solved 1. Define the following fluid properties : Density