FDA Approves Senza®, Nevro's High Frequency Spinal Cord
The Senza System has been approved by the FDA for the treatment of chronic pain associated with painful diabetic neuropathy.
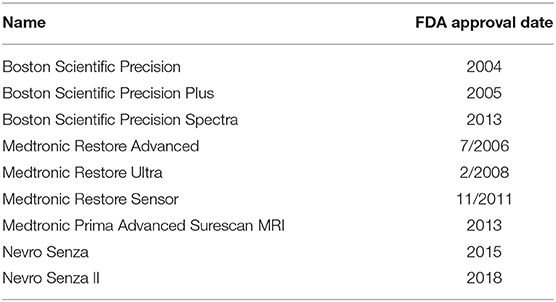
Frontiers Survey of Spinal Cord Stimulation Hardware Currently Available for the Treatment of Chronic Pain in the United States

Senza Spinal Cord Stimulation (SCS) System – P130022/S042

Nevro Announces U.S. Full Market Launch of Revolutionary HFX iQ™ Spinal Cord Stimulation System to Personalize Treatment of Chronic Pain

Nevro announces FDA approval of Senza SCS system for PDN
Senza Archives - Beyond Type 1

Nevro Receives FDA Approval For Senza II Spinal Cord Stimulation System Delivering HF10 Therapy

Nevro Corp. - Nevro Announces FDA Approval of its 10 kHz High Frequency Spinal Cord Stimulation Therapy for Treatment of Chronic Pain Associated with Painful Diabetic Neuropathy (PDN)

Nevro Corp.

FDA approves Nevro's Senza system to treat chronic pain with diabetic neuropathy - MassDevice









