The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens
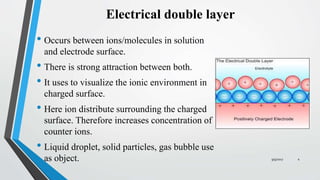
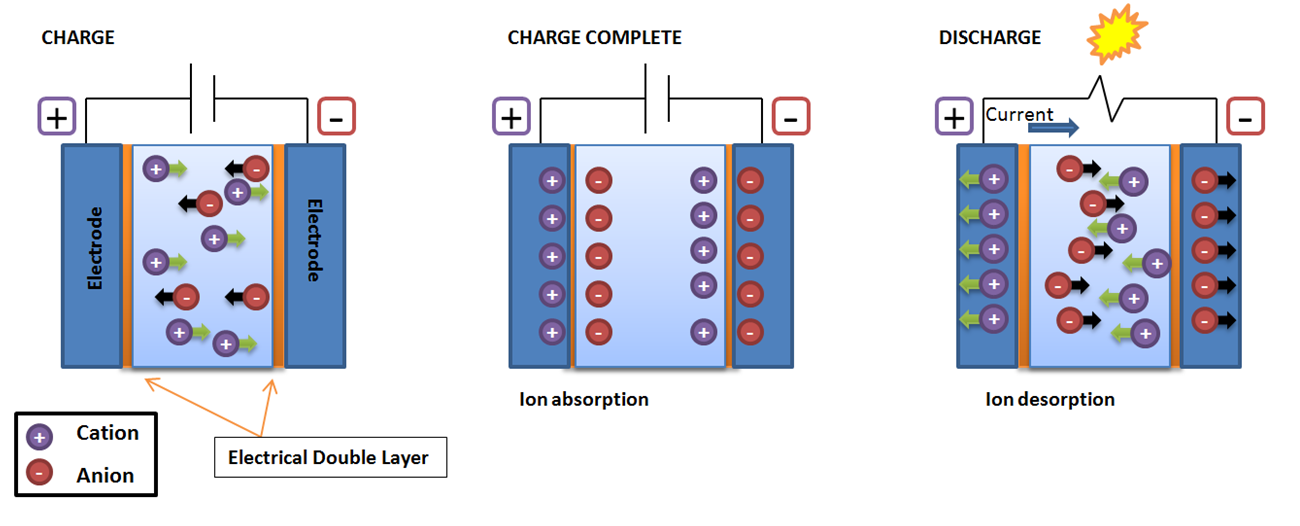
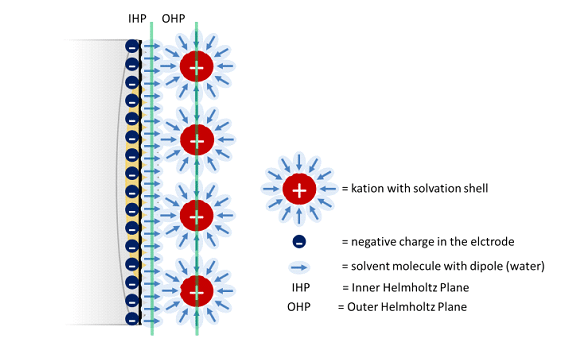
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
Electrochemical Impedance Spectroscopy - Engineering LibreTexts

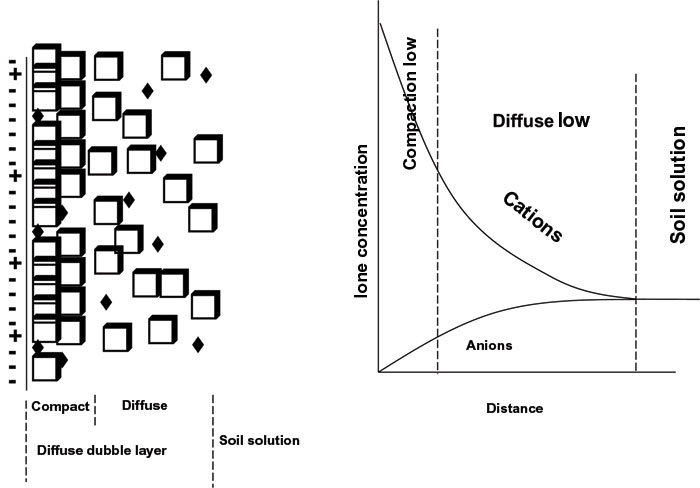
The Grahame model of the metal electrode/electrolyte double layer

PDF) Cottrell's equation revisited: An intuitive, but unreliable
The Cottrell Experiment and Diffusion Limitation 2/3 - The

Emergence of a Stern Layer from the Incorporation of Hydration

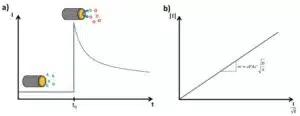
Cottrell Equation

Chronoamperometry - Electrochemistry Flashcards

PDF) Bioinspired Chemically Modified Electrodes for Electroanalysis

Electric Double Layer - an overview
The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens