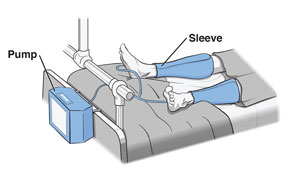
AIROS Medical Receives FDA Clearance to Market New Peristaltic
AIROS Medical announces FDA 510k clearance to market the AIROS 8P compression device and Pants garment that treats leg and pelvic swelling.

Hei-VAP Industrial Rotary Evaporators, Heidolph®

Used WATSON-MARLOW 6000 Peristaltic Pump For Sale - DOTmed Listing

AIROS Medical granted FDA 510k clearance to market compression device for Lymphedema treatment - NS Medical Devices

AIROS Medical Granted U.S. Trademark Registration for Company

Laura Wright على LinkedIn: Top 5 Questions and Answers about Lymphedema Nutrition

Occupational Therapy Software Market Trends And Forecast 2033

Compression Therapy Device Technology for Lymphedema Treatment

Laura Wright على LinkedIn: Top 5 Questions and Answers about Lymphedema Nutrition

Sitemap - AIROS Medical, Inc.

FDA Advisory No.2022-2056

Airlife 2270135 - McKesson Medical-Surgical

AIROS Medical Receives FDA Clearance to Market New Peristaltic Pneumatic Compression Device, Truncal Garments for Lymphedema Treatment

AIROS Medical (@AirosMedical) / X

Laura Wright على LinkedIn: Top 5 Questions and Answers about Lymphedema Nutrition

New AVANOS MEDICAL 7180-20 MIC Safety Percutaneous Endoscopic Gastrostomy (PEG) Kit , 20Fr , Type: Pull (Expired) Disposables - General For Sale - DOTmed Listing #4618512