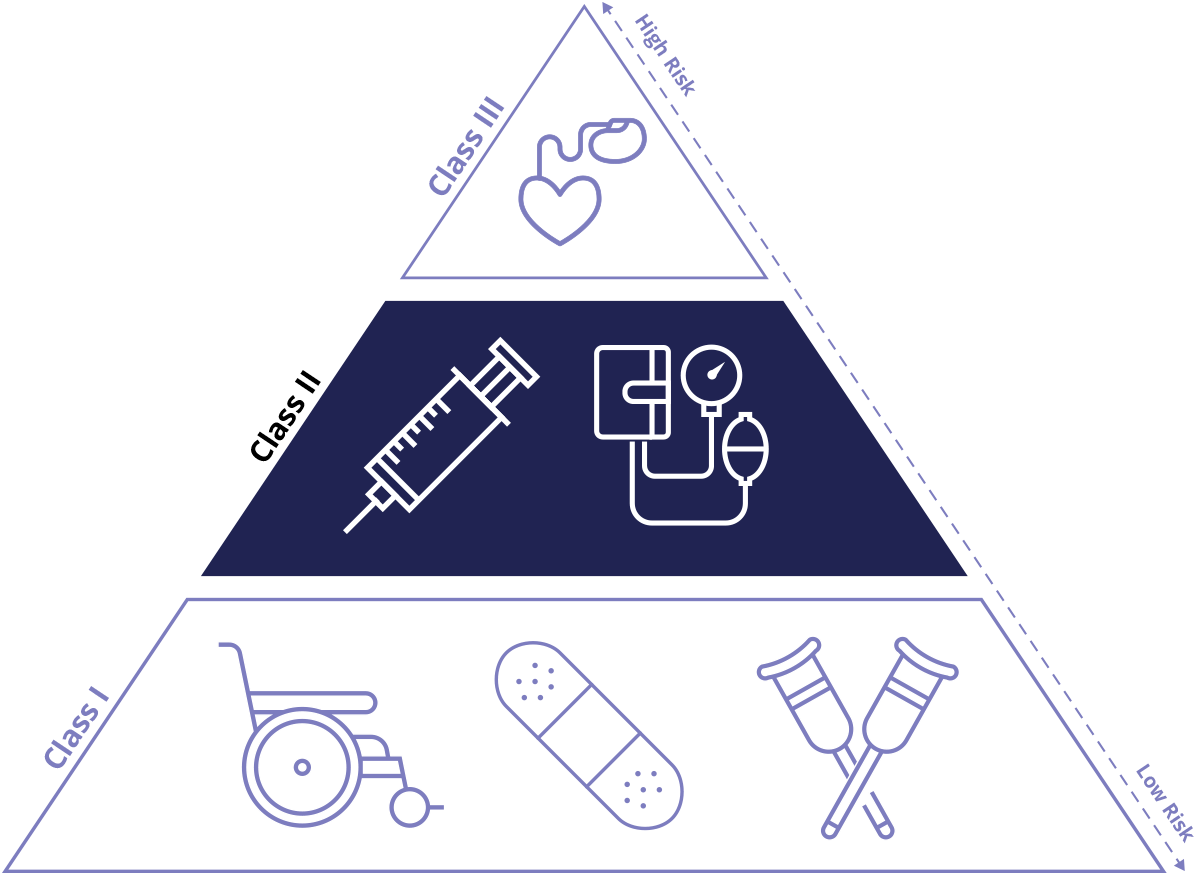
Class II Device Definition
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.

Risk Classification of Medical Devices in the United States and Europe

SOCRA CCRP Exam - Term: Definition: What is in 21 CFR Part 11
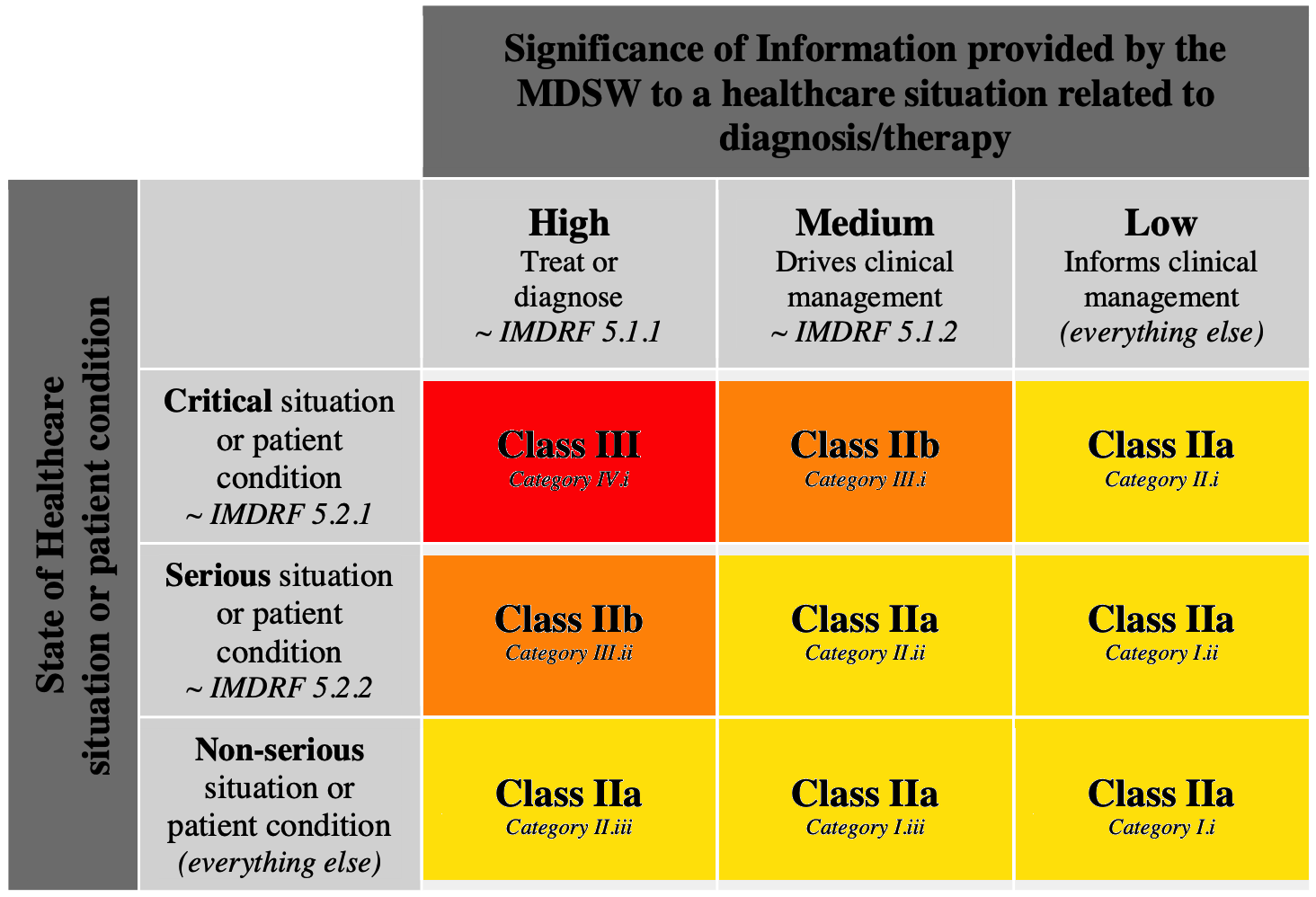
How to qualify, classify and CE mark software - Software in Medical Devices, by MD101 Consulting

Medical Device Approval Processes in United States
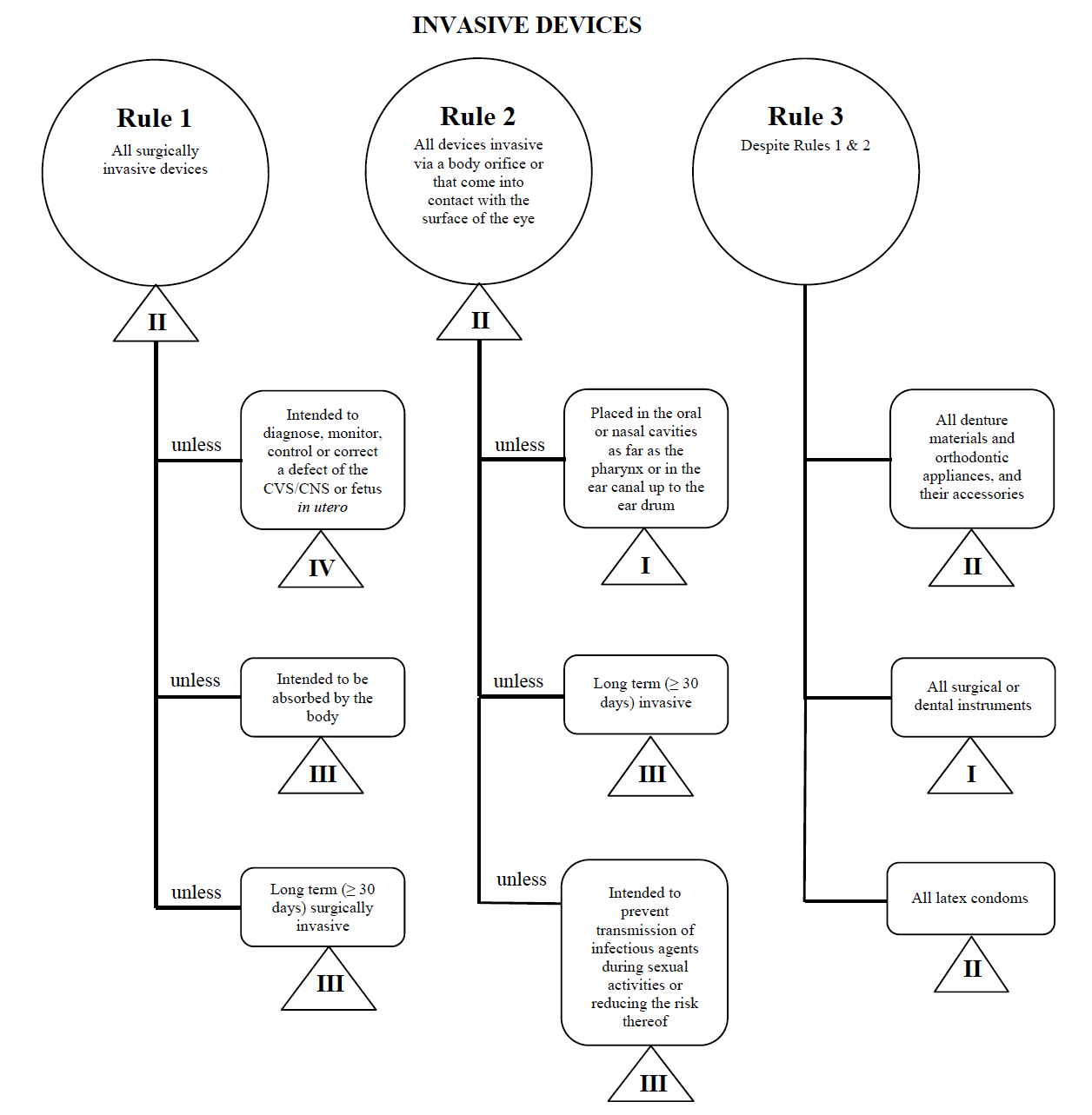
Medical Device Classification Guide - How To Determine Your Device Class

FDA Class II medical devices

Medical Device Classification (FDA)

MDR - Classes and Conformity - tracekey solutions GmbH

Never accept the mark of the beast

FDA Class 2 Medical Device Overview
Classify Your Medical Device

De Novo classification process: a beginner's guide
SOCRA - CCRP Exam with Questions and Answers 2024

Medical device regulations, classification & submissions
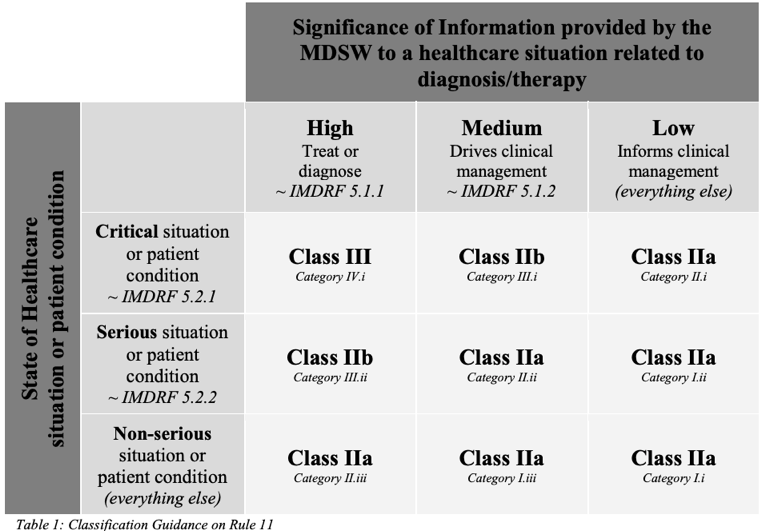
Medical device software (MDSW) under EU MDR and IVDR