
Calculate the number of molecules of CO_2 present in 4.4 g of it.
Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of molecules of co2 present in 44 g of it
Click here👆to get an answer to your question ✍️ Calculate the number of molecules of CO-2 present in 4-4 g of it

A flask contains 4.4 gm of CO2 gas .calculate- 1. how many moles of CO2 gas are present in the sample? 2.

How to Calculate Percent Yield in Chemistry: 15 Steps

Plz Tell the 15th question Q 15 A flask contains 4 4 g of CO2 gas Calculate - Science - Atoms and Molecules - 12378457
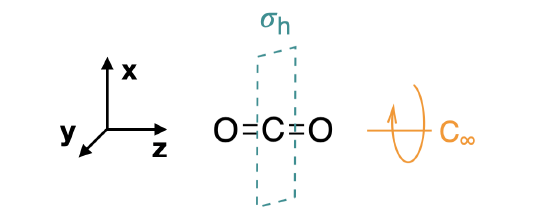
5.4.2: Carbon Dioxide - Chemistry LibreTexts
What is the mass of 2.24 litres of CO2 gas at STP? - Quora

5 2.0 YUI marsn gas 42 Tv.v Bohosgene 23. The number of molecules present in 4.4g of CO, gas is [Jipmer-1990] 1) 6.023x1023 2) 5.023x102 3) 6.023x1024 4) 6.023x1022 of 1) +2 .

Mole Concept 28. How many electrons are present in 180gm. of water

Calculate (i) number of molecules present in 2.24 dm^3 of carbon dioxi
31. which of the folowing has lowest weight? 1) 6.023 x 1022 molecules of glucose 2) 18ml of water at 40C 3) 11200ml of CH4 at stp 4) 5.6 litre of CO2 at stp

Solved a. Calculate the number of atoms of each element









