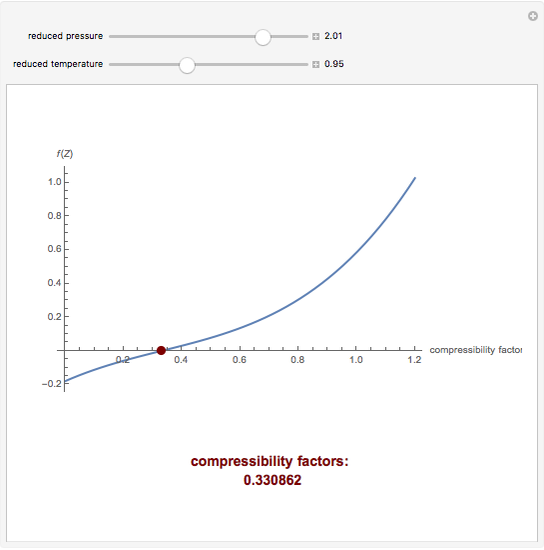
At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question
Solved Please answer all the questions and explain how the
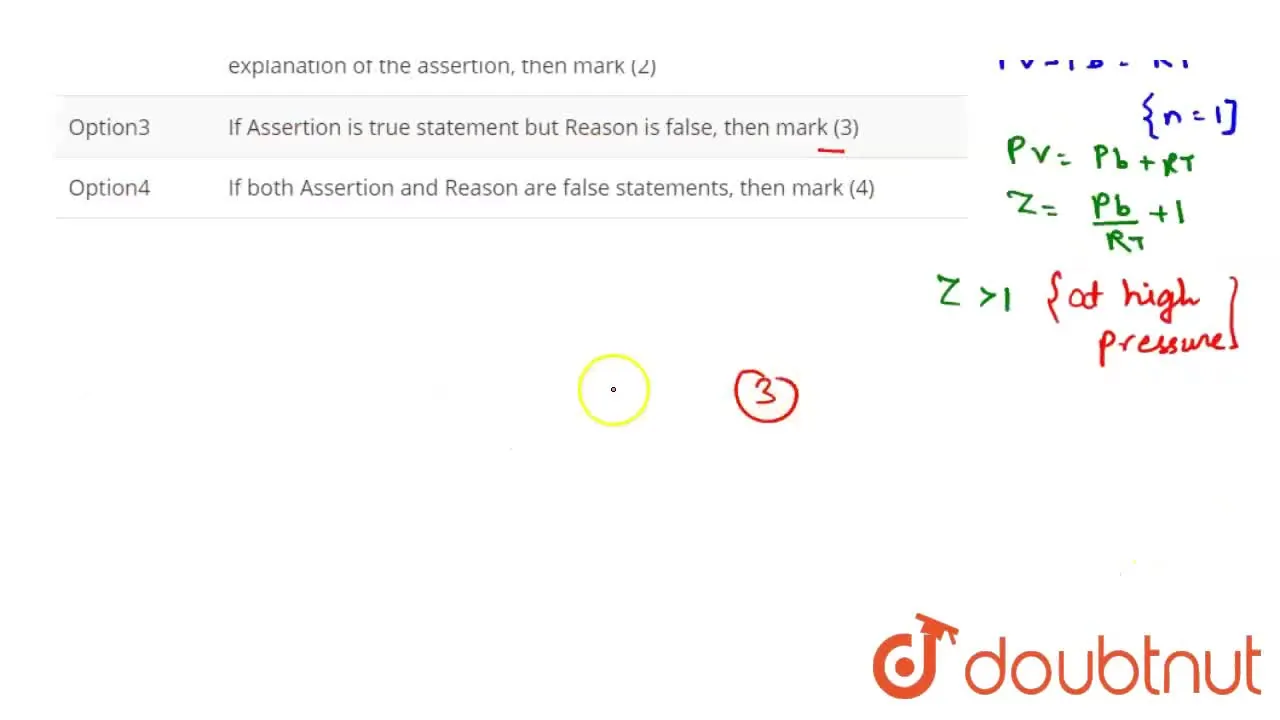
If Assertion is true statement but Reason is false, then mark (3)

Solved 3.91. The definition of compressibility factor Z, Eq.

1 The Ideal Gas. 2 Ideal gas equation of state Property tables provide very accurate information about the properties. It is desirable to have simple. - ppt download

Gaseous State Questions for JEE exam - Free Online All questions of Gaseous State - Chapter-wise Questions of JEE

If Z is a compressibility factor, van der Waals equation at low pressure can be written as [JEE
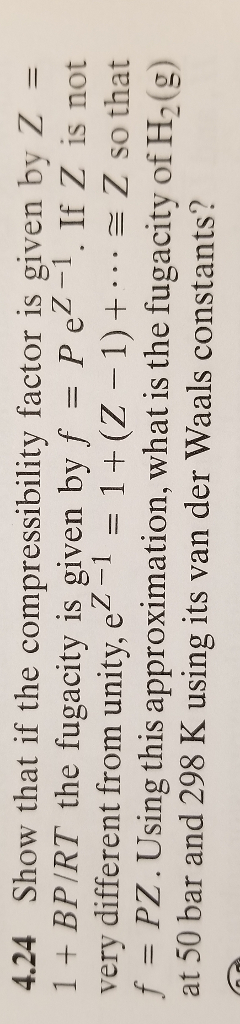
Solved 4.24 Show that if the compressibility factor is given

The compressibility factor(s) for an ideal gas is/are: (A) unity

If `Z` is a compressibility factor, van der Waals' equation at low

The compressibility factor a real gas high pressure is:1+ dfrac{RT}{pb}1+ dfrac{pb}{RT}11- dfrac{pb}{RT}