The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8


my lioperties, Abnormality in Molar Mass) 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g vapour pressure =

Solutions (1-47) - Final, PDF, Solubility
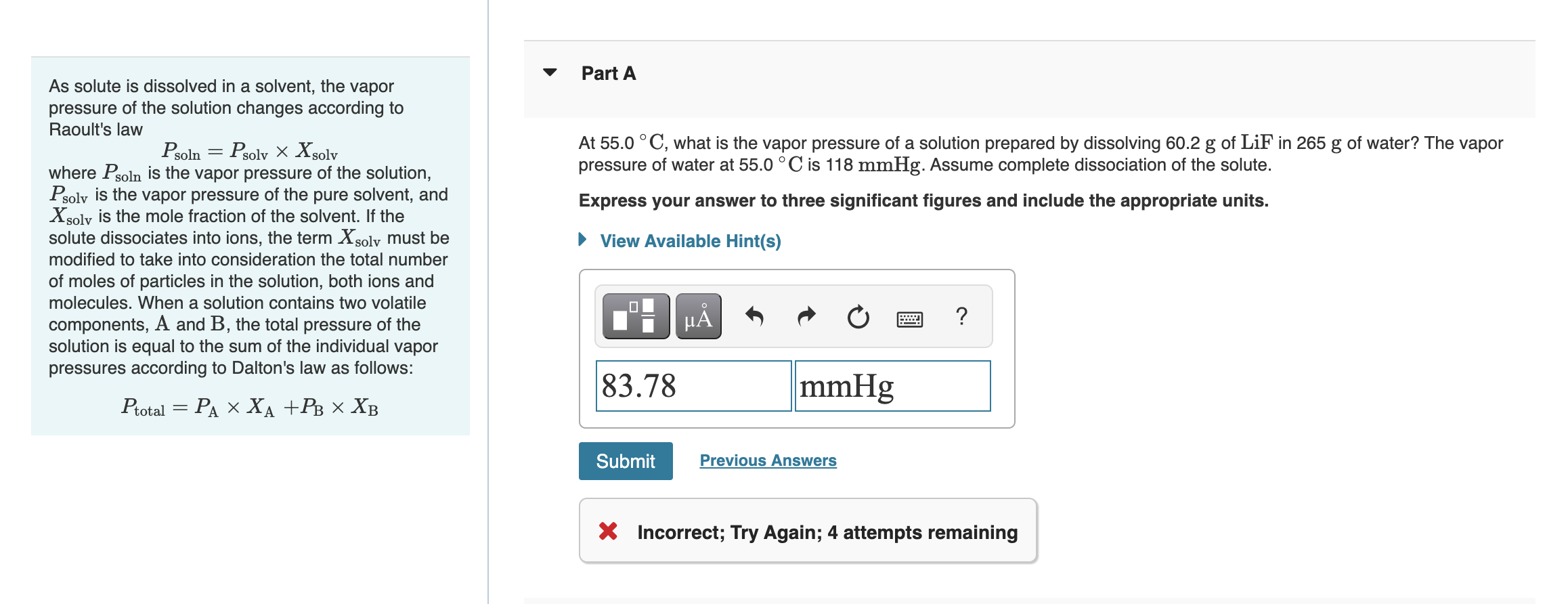
Solved As solute is dissolved in a solvent, the vapor
Stoichiometry Practice 2 answer key

Chapter-10 Solutions.pdf - Chemistry - Notes - Teachmint

IIT JEE Main Complete Chemistry.pdf
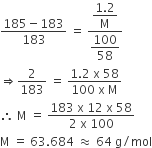
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o

Solutions (1-47) - Final, PDF, Solubility

LOPOUL 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is








